Abstract
Pre-communicating or A1 segment of anterior cerebral artery (A1ACA) hypoplasia can negotiate the anterior cerebral circulation. Not many studies have been examined the association of hypoplastic A1ACA and cerebral ischemic stroke (CIS). In this study the authors' want to accomplish the relationship between hypoplastic A1ACA and outcomes among the patients with CIS in Andhra Pradesh population of India. Retrospective review of prospectively identified 201 adult patients with CIS from 2015 to 2017 was achieved. Patients underwent 3.0T intracranial magnetic resonance angiography were compared with clinical and radiological aspects between male and female cases of A1ACA hypoplasia with associated variations in the circle of Willis. The obtained data was statistically analysed using SPSS software version 16.0 for Windows and P-value <0.05 was considered as significant. Chi-square test was applied to find out the association between the sex and incidence of hypoplastic A1ACA. Sixty-four of 201 patients with A1ACA hypoplasia with no aplastic cases were recorded. It was found to be more in males than females and common on right than left side. Frequent neurological indications such as headache, dizziness, visual instability, nausea, weakness of extremities and seizure were noted and most cases were associated with CIS. Hypoplastic A1ACA often associated with ischemia of terminal branches of ipsilateral ACA which is compromised by the blood flow via contralateral ACA. In this study, though the CIS is not directly related to hypoplastic A1ACA, any alterations in A1 segment is a considerable risk factor of stroke.
Internal carotid arteries (ICA) and vertebral arteries supply human brain bilaterally and anastamose to form circulus arteriosus or circle of Willi's (CW) in the interpeduncular fossa at the base of brain [1]. Like any other part of arterial system, the CW also shows many anatomical variations in the cranial cavity and approximately only 42% of humans hold complete CW [2].
The anterior part of CW provide anterior collateral circulation through the bilateral pre-communicating (A1 segments) anterior cerebral arteries (ACA) with anterior communicating artery (AcomA) and posterior collateral circulation through the bilateral pre-communicating (P1 segments) posterior cerebral arteries (PCA) with posterior communicating artery [34567].
The commonest type of anatomical variation in the CW is hypoplastic or aplastic A1 segment of ACA (A1ACA) [8] and the incidence ranging from 1% to 16% observed in angiograms, autopsy reports and anatomical dissections [910].
The ACA is one of the terminal branches of ICA and it is communicated with fellow of opposite side through AcomA. The ACA is divided into five segments. A1 segment is its origin from ICA to AcomA; A2 segment, from AcomA to the origin of callosomarginal artery; A3 segment, distal to the origin of callosomarginal artery; and A4 and A5 are the smallest branches and are known as callosal arteries [111213].
To our knowledge, literature documentation of the incidence of hypoplastic or aplastic A1ACA is scanty in southern part of India. The aim of this study is to provide anatomic information and to delineate the range of A1ACA hypoplasia or aplasia in Andhra population of India using magnetic resonance angiography (MRA) of CW. This collected retrospective data from single centre study can be correlated with the clinical characteristic features of the stroke patients which can provide additional information in the diagnosis and treatment of cerebral infarction.
The present study was a retrospective analysis of MRA of CW on 201 patients, which included 132 males and 69 females with the age range of 24–79 years. The cases were obtained between the years 2015–2017 from patients presented with symptoms of cerebral ischemic stroke (CIS) at the Narayana General Hospital, Nellore, Andhra Pradesh, India. A time of flight (TOF)-MRA technique was used and the study was conducted and analyzed in the departments of radiology, anatomy and neurology. All the patients underwent 3D TOF-MRA using 3.0T MRI machine (3.0T system, GE Discovery MR750w 3.0Tesla, GE Healthcare, Milwaukee, WI, USA) and imaging parameters used were (1) repetition time was 30 milliseconds and echo time was 2.7–3.1 milliseconds, (2) flip angle was 20°, (3) 200 mm field of view, (4) section thickness was 1.4 mm, and (5) the imaging time was approximately 4.49 minutes. The images obtained were processed using a maximum intensity projection algorithm to create an angiogram like image. The reconstructed images were then analyzed to detect the hypoplastic (<1 mm in diameter) or aplastic A1ACA [14]. Cases with stenosis of ICA with hypoplastic A1ACA, aneurysm of ICA or ACA and fenestrated ACA were excluded from this study. The study was approved by the institutional ethical committee and clinical variables were abstracted from Institutional Neurology review board. The statistical analysis was done with SPSS software version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) and P-value >0.05 was considered as statistically significant. Chi-square test was applied to find out the association between the sex and incidence of hypoplastic A1ACA.
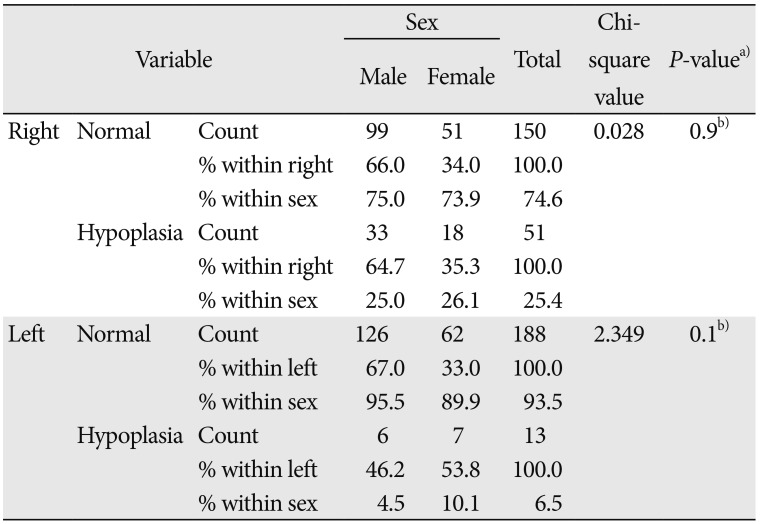
The present study revealed the incidence of hypoplastic A1ACA using cerebral MRA (Fig. 1A, B). The mean age of the patients were found to be 57.64±11.74 in males and 56.17±13.11 in females (Table 1). The MRA was performed within 72 hours of ischemic stroke onset. Out of 201 cases, 64 cases (31.9%) presented ipsilateral hypoplastic A1ACA which includes 51 on right (25.4%) and 13 cases on left side (6.5%).
Out of 64 totally observed incident cases of hypoplastic A1ACA, 39 (60.94%) were males, which included 33 on right side (64.7%) and six on left side (46.2%). Similarly the females were 25 (39.06%) cases, included 18 on right side (35.3%) and 7 on left side (53.8%) (Table 2). No cases of bilateral hypoplasia of A1ACA and aplastic A1 segment on right or left sides or both were observed.
Out of 64 cases of hypoplastic A1ACA, 46 cases (71.88%) had ipsilateral ischemia and five (7.81%) presented with bilateral ischemia on terminal branches of ACA which appears to be the risk factor contributing to ischemic stroke, but majority of them had first-ever transient ischemic attack (n=33, 71.74%). The remaining 13 hypoplastic A1ACA (20.31%) had no stroke symptoms from ACA involvement.
Among the 64 hypoplastic A1ACA cases, five of them on right side (7.81%) in addition showed fetal-type posterior cerebral circulation for total absence of right P1 segment of PCA (Fig. 2A). In these cases, ipsilateral weakness of lower and upper extremities, loss of sensation, and slurred speech were also been observed. One of the female case on left side, demonstrated the hypoplastic A1, 2, and 3 segments of ACA and no blood flow related signal was noted, diagnosed it as complete hypoplasia of ACA (Fig. 2B).
The commonest neurological symptoms noted in male and female cases were chronic headache and dizziness (n=43, 67.19%), visual instability (n=14, 21.87%), nausea (n=12, 18.75%), weakness of extremities (n=9, 14.06%), and seizure (n=8, 12.5%).
The A1ACA is the primary supplier of anterior cerebral collateral blood flow. Hypoplastic or aplastic A1ACA is an exceptional fetal variant of the CW. Absence or aplastic A1ACA is a rare variation of anterior cerebral circulation and its occurrence was 1%–4.8% but hypoplastic A1 segment was more frequently reported between 1% and 13% of general population [38151617] and it was 31.9% in present study. Many cases in this study showed right side hypoplasia of A1ACA (25.4%) and these observations were coinciding with the other findings [181920].
Majority of cases (64.06%) in the present study linked circulation from contralateral A1 segment of ICA was exposed to poor blood supply to ipsilateral or bilateral striatal areas supplied by terminal striatal branches of ACA leads into hypoplastic related strokes [41921]. On the contrary, this persistent single robust A1 segment of ICA serves blood to both ACA territory of cerebrum which may not compensate fully to both hemispheres may leads to stroke as seen in our study which was agreeing with earlier studies [2223].
In the present study, right A1ACA hypoplasia associated ipsilateral fetal variant of PCA showed the ataxic hemiparesis indicated a less effective collateral blood supply by CW leads to increased occurrence of anterior or posterior cerebral infarction [24].
According to the literature the hypoplasia or aplasia in the segments of ACA is commonly associated with the presence of carotid artery stenosis [2526] and no such cases were documented in this current study.
In this study, there is no implication between the presence or absence of hypoplastic A1 segment and anterior cerebral stroke, but often associated with ischemia of terminal branches of ipsilateral ACA which was compromised by contralateral ACA [19]. In general most of the hypoplastic or aplastic A1ACA is asymptomatic and it is not directly linked with any particular neurological disease but may cause inter or intrahemispheric collateral circulation failure which may be the increase risk of stroke in the ACA province [27]. In 2011, Han et al. [28] observed that the hypoplastic A1ACA in pediatric population was associated predominantly with headache and dizziness, which may play a role in the incidence of neurological diseases in their later life. So the clinicians or neurologists working in this area should be aware of hypoplastic A1ACA that may lead into progressive cerebral ischemia and can necessitate precautionary rehabilitation and treatments accordingly.
The limitations of this study is smaller sample size, so further studies on hypoplastic A1ACA is required to understand its presumption in large-scale. Despite these limitations, the authors believe that CIS associated with hypoplasia of A1ACA is a valid ground as observed from the present study.
To our comprehension, this is the first study on patients in Andhra Pradesh population of India with hypoplastic A1ACA using 3.0T MRA. These findings are in line with preceding studies which signifies that a compromised anterior collateral circulation through the CW may play an important role in patients with cerebral stroke.
References
1. Orlandini GE, Ruggiero C, Orlandini SZ, Gulisano M. Blood vessel size of circulus arteriosus cerebri (circle of Willis): a statistical research on 100 human subjects. Acta Anat (Basel). 1985; 123:72–76. PMID: 4050309.
2. Krabbe-Hartkamp MJ, van der, de Leeuw FE, de Groot JC, Algra A, Hillen B, Breteler MM, Mali WP. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998; 207:103–111. PMID: 9530305.
3. Chuang YM, Liu CY, Pan PJ, Lin CP. Anterior cerebral artery A1 segment hypoplasia may contribute to A1 hypoplasia syndrome. Eur Neurol. 2007; 57:208–211. PMID: 17268201.
4. Kang DW, Chu K, Ko SB, Kwon SJ, Yoon BW, Roh JK. Lesion patterns and mechanism of ischemia in internal carotid artery disease: a diffusion-weighted imaging study. Arch Neurol. 2002; 59:1577–1582. PMID: 12374495.
5. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998; 55:1475–1482. PMID: 9823834.
6. Wang CX, Todd KG, Yang Y, Gordon T, Shuaib A. Patency of cerebral microvessels after focal embolic stroke in the rat. J Cereb Blood Flow Metab. 2001; 21:413–421. PMID: 11323527.
7. Liebeskind DS. Collateral circulation. Stroke. 2003; 34:2279–2284. PMID: 12881609.
8. Uchino A, Nomiyama K, Takase Y, Kudo S. Anterior cerebral artery variations detected by MR angiography. Neuroradiology. 2006; 48:647–652. PMID: 16786350.
9. Stefani MA, Schneider FL, Marrone AC, Severino AG, Jackowski AP, Wallace MC. Anatomic variations of anterior cerebral artery cortical branches. Clin Anat. 2000; 13:231–236. PMID: 10873213.
10. Gunnal SA, Wabale RN, Farooqui MS. Variations of anterior cerebral artery in human cadavers. Neurol Asia. 2013; 18:249–259.
11. Ugur HC, Kahilogullari G, Esmer AF, Comert A, Odabasi AB, Tekdemir I, Elhan A, Kanpolat Y. A neurosurgical view of anatomical variations of the distal anterior cerebral artery: an anatomical study. J Neurosurg. 2006; 104:278–284. PMID: 16509502.
12. Lehecka M, Dashti R, Hernesniemi J, Niemelä M, Koivisto T, Ronkainen A, Rinne J, Jääskeläinen J. Microneurosurgical management of aneurysms at A3 segment of anterior cerebral artery. Surg Neurol. 2008; 70:135–151. PMID: 18482754.
13. Steven DA, Ferguson GG. Winn HR, editor. Distal anterior cerebral artery aneurysms. Philadelphia, PA: W.B. Saunders Co.;2004. p. 1945–1957.
14. Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994; 330:1565–1570. PMID: 8177246.
15. Maurer J, Maurer E, Perneczky A. Surgically verified variations in the A1 segment of the anterior cerebral artery. Report of two cases. J Neurosurg. 1991; 75:950–953. PMID: 1823542.
16. Niederberger E, Gauvrit JY, Morandi X, Carsin-Nicol B, Gauthier T, Ferré JC. Anatomic variants of the anterior part of the cerebral arterial circle at multidetector computed tomography angiography. J Neuroradiol. 2010; 37:139–147. PMID: 20346510.
17. Ansari S, Dadmehr M, Eftekhar B, McConnell DJ, Ganji S, Azari H, Kamali-Ardakani S, Hoh BL, Mocco J. A simple technique for morphological measurement of cerebral arterial circle variations using public domain software (Osiris). Anat Cell Biol. 2011; 44:324–330. PMID: 22254161.
18. De Silva KR, Silva R, Gunasekera WS, Jayesekera RW. Prevalence of typical circle of Willis and the variation in the anterior communicating artery: a study of a Sri Lankan population. Ann Indian Acad Neurol. 2009; 12:157–161. PMID: 20174495.
19. Shaban A, Albright K, Gouse B, George A, Monlezun D, Boehme A, Beasley TM, Martin-Schild S. The impact of absent A1 segment on ischemic stroke characteristics and outcomes. J Stroke Cerebrovasc Dis. 2015; 24:171–175. PMID: 25440333.
20. Lakhotia M, Pahadiya HR, Prajapati GR, Choudhary A, Gandhi R, Jangid H. A case of anterior cerebral artery A1 segment hypoplasia syndrome presenting with right lower limb monoplegia, abulia, and urinary incontinence. J Neurosci Rural Pract. 2016; 7:189–191. PMID: 26933381.
21. Honig A, Eliahou R, Auriel E. Confined anterior cerebral artery infarction manifesting as isolated unilateral axial weakness. J Neurol Sci. 2017; 373:18–20. PMID: 28131184.
22. Dimmick SJ, Faulder KC. Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics. 2009; 29:1027–1043. PMID: 19605654.
23. Okahara M, Kiyosue H, Mori H, Tanoue S, Sainou M, Nagatomi H. Anatomic variations of the cerebral arteries and their embryology: a pictorial review. Eur Radiol. 2002; 12:2548–2561. PMID: 12271398.
24. Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000; 31:128–132. PMID: 10625727.
25. Hendrikse J, Eikelboom BC, van der Grond J. Magnetic resonance angiography of collateral compensation in asymptomatic and symptomatic internal carotid artery stenosis. J Vasc Surg. 2002; 36:799–805. PMID: 12368718.
26. Hoksbergen AW, Legemate DA, Csiba L, Csáti G, Síró P, Fülesdi B. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis. 2003; 16:191–198. PMID: 12865604.
27. Gerstner E, Liberato B, Wright CB. Bi-hemispheric anterior cerebral artery with drop attacks and limb shaking TIAs. Neurology. 2005; 65:174. PMID: 16009921.
28. Han YK, Kim S, Yoon CS, Lee YM, Kang HC, Lee JS, Kim HD. A1 segment hypoplasia/aplasia detected by magnetic resonance angiography in neuropediatric patients. J Korean Child Neurol Soc. 2011; 19:231–239.
Fig. 1
(A, B) Cerebral magnetic resonance angiogram showing the right and left hypoplasia of A1 segment of anterior cerebral artery (arrow), respectively.
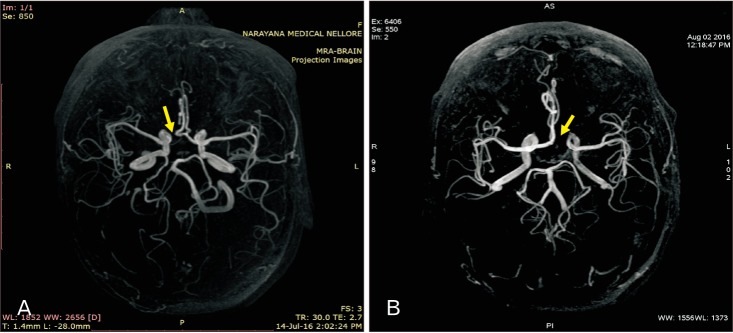
Fig. 2
(A) Cerebral magnetic resonance angiography (MRA) showing the right hypoplastic A1 segment of anterior cerebral artery (arrow) with fetal-type posterior cerebral circulation for total absence of right P1 segment of posterior cerebral arteries (arrowhead). (B) MRA showing the hypoplastic A1, 2, and 3 segments of anterior cerebral artery (ACA) (arrow) and no blood flow related signal was noted, identified it as complete hypoplasia of ACA.
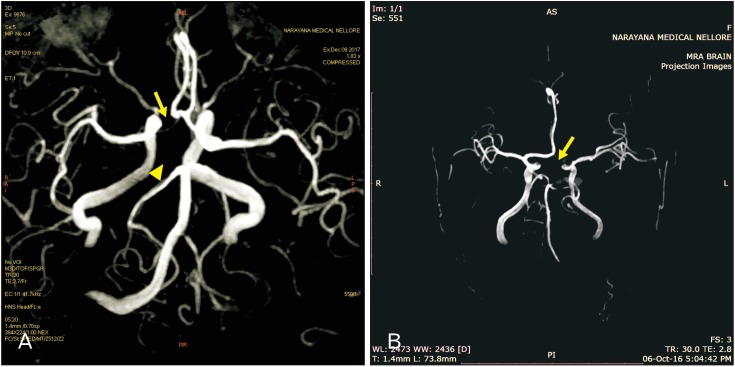
Table 1
Demographic data of patients studied in relation to sex and age
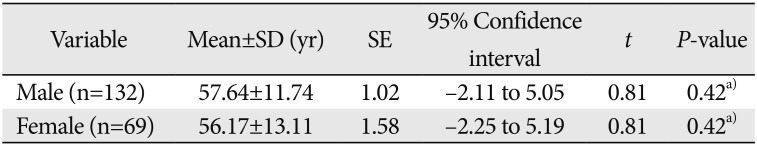
| Variable | Mean±SD (yr) | SE | 95% Confidence interval | t | P-value |
|---|---|---|---|---|---|
| Male (n=132) | 57.64±11.74 | 1.02 | −2.11 to 5.05 | 0.81 | 0.42a) |
| Female (n=69) | 56.17±13.11 | 1.58 | −2.25 to 5.19 | 0.81 | 0.42a) |
Table 2
Side and sex-specific incidence of hypoplastic A1 segment of anterior cerebral artery




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download