Abstract
Because of its embryonic origin, the thyroid gland is predisposed to multiple anatomical variations and developmental anomalies. These include the pyramidal lobe, the origin of levator glandular thyroidae, the absence of the isthmus, ectopic thyroid, accessory thyroid tissues, etc. These anatomical variations are clinically significant to surgeons, anatomists, and researchers. The present study was designed to report anatomical variations and developmental anomalies of the thyroid gland in Ethiopian population. The study was conducted on 40 cadavers used for routine dissection classes. The thyroid gland was exposed and observed for any variations and developmental anomalies. The length, width, and thickness of the lobes were measured using a vernier caliper. Differences in the incidence of pyramidal lobe and absence of the isthmus between sexes were tested using a Pearson chi-square test. The mean length, width, and thickness of the right lobe were 4.24 cm, 1.8 cm, and 1.6 cm, respectively, whereas it was 4.08 cm, 1.8 cm, and 1.6 cm, respectively for that of the left lobe. The pyramidal lobe was noted in 52.5% of the cadavers. The levator glandulae thyroidae were prevalent in 40% of the cadavers. The isthmus mainly overlies the 2nd to 4th tracheal rings and was absent in 7.5% of the cadavers. Accessory thyroid tissue and double pyramidal lobes were noted in 2.5% of the cadavers. Most of the variations of the thyroid gland were seen frequently in female but it was not statically significant. Different clinically important and rare variations of the thyroid gland were found.
The thyroid gland is a butterfly shaped endocrine gland concerned with regulating basal metabolic rate, blood calcium level and growth and development in mammals [12]. It is situated anteriorly in the neck at the level of the fifth cervical to first thoracic vertebrae deep to the platysma, sternothyroid, and sternohyoid muscles [345]. It has two lateral lobes connected by a narrow median bridge called isthmus [5]. The normal size of the lobes of the thyroid gland has been described to be 5 cm long, 3 cm wide, and 2 cm thick [56]. The thyroid size, shape, and volume vary with age, race, geographical location, and sex [678].
Of all endocrine glands, the thyroid is the first to develop in the embryo [3910]. It begins to develop as a median thickening of endoderm on the floor of the pharynx between the first and the second pharyngeal pouches during the fourth week of gestation [6911]. As the embryo and tongue grow, the developing thyroid gland descends in the neck, passing ventral to the developing hyoid bone and laryngeal cartilages [612]. For a short time, the thyroid gland is connected to the tongue by a narrow tube, the thyroglossal duct [12]. At first, the thyroid primordium is hollow, but soon becomes a solid mass of cells and divides into right and left lobes that are connected by the isthmus which lies anterior to second, third, and fourth tracheal rings [13612]. The thyroid gland has assumed its definitive shape and reaches its place below the thyroid cartilage by end of the seventh week [612]. The duct becomes obliterated by the eighth to the 10th weeks of gestation [6].
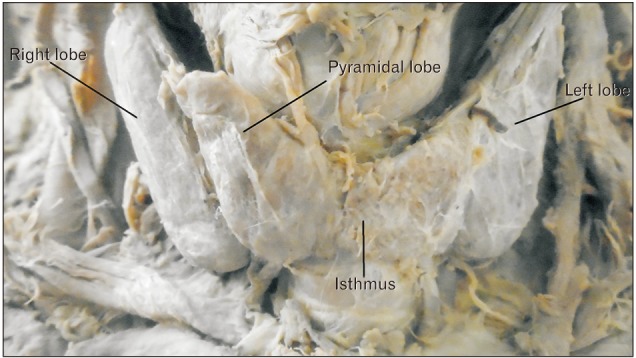
Because of its embryonic origin the thyroid gland is predisposed to wide range of multiple anatomical variations and developmental anomalies including, pyramidal lobe (PL) or lobe of Lalouette, origin of levator glandulae thyroidae (LGT), absence of isthmus, agenesis, ectopic thyroid, accessory thyroid tissue, and thyroglossal duct cysts [2349111314]. Most of these variations of the thyroid gland are due to a persistence of the thyroglossal duct [9]. The PL develops from the distal part of the thyroglossal duct by differentiation of the duct tissue into glandular tissue which is attached by its base to the superior border of isthmus usually at its junction with the left lobe [12111315]. The incidence of PL varies from 15% to 75% [16].
The apex of PL is attached to the body of the hyoid bone or thyroid cartilage by LGT [1491315]. LGT is classified into five types: hyopyramidalis, thyreopyramidalis, thyreoglandularis, hyoglandularis, and tracheoglandularis, according to their attachments [14]. The agenesis of the isthmus is characterized by the absence of isthmus with lateral lobes positioned independently on either side of the trachea [6].
The PL is a frequent site of recurrent thyroid diseases after thyroidectomy and is mainly affected by primary thyroid disease such as multinodular goiter, Hashimoto thyroiditis, and Grave diseases [1617]. The PL also affects follow-up of patient after thyroidectomy: in a patient who will require postoperative radioactive iodine, the PL absorbs most of the radioactive agent and almost nullify the anticipated benefit of 131I [1618]. The presence of PL also affects sensitivity of serum thyroglobulin measurement after thyroidectomy [19].
Many studies reveal that thyroid diseases are a common cause of illness and reason for seeking medical care in Ethiopia [202122]. In one study conducted in Ethiopia, thyroid disorders accounts 59.6% of endocrine disorders [21]. Thyroidectomy and tracheostomy are among the commonest clinical practice in Ethiopia to treat thyroid diseases and their complications [21]. Anatomical variations of the gland such as PL, ectopic thyroid, accessory thyroid tissue, etc. can cause complication of thyroid surgery [9]. The superb knowledge about the normal anatomy and variations of structures is very important in evaluation and successful surgical management of diseases in clinical practice [23]. Age, sex, and race of population studied, all of which can contribute to the anatomical variations of the thyroid gland [24]. There was no published report about the anatomical variations of the thyroid gland among Ethiopian people. Thus, the present study was designed to report the prevalence of anatomical variations and developmental anomalies of the thyroid gland among Ethiopian cadavers that will hopefully give information to surgeons, anatomists and researchers.
The descriptive cross-sectional study was conducted on 40 Ethiopian cadavers used for routine dissection classes for medical undergraduate and anatomy postgraduate students. The study was conducted on cadavers available at the University of Gondar, Bahirdar University, Debre Markos University, Addis Ababa University, Jimma University, Arba Minch University, Sante Medical College, Bethel Medical College, Saint Paulos Millennium Medical College, Hayat Medical College, Gamby Medical College, and Wachemo University from May to September 2017. All cadavers available at mentioned medical schools in Ethiopia in the study period were the study population. Cadavers who were normal and free from any gross pathology were included in the study. The cadavers with gross pathology, including goiter and destruction on the neck region were excluded.
A checklist was prepared and used for data collection. To insure the quality of data, both interobserver and intraobserver variability assessment was carried out where two observers were involved. The data were collected by anatomists. The neck region of the cadavers was dissected according to Cunningham's practical manual of anatomy to expose the thyroid gland. The dissected region was carefully observed for any variations and developmental anomalies of the thyroid gland, including partial and total agenesis of the gland, presence of ectopic tissues or accessory thyroids, permanent thyroglossal duct anomalies such as a cyst, fistula, sinus, and PL and LGT. The length, width, and thickness of the lateral lobes and if present the PL was measured using a vernier caliper. The length of the lateral lobes was measured in the cranial and caudal poles; the width and thickness were taken from the middle of the lobes. The length of the PL was measured from the base to the apex, the width was taken as the transverse diameter of the base and thickness was the anteroposterior diameter of the base. The isthmus relation to the tracheal rings was noted. The specimens were photographed and the findings were appropriately documented. Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA) after exporting the data from Epi Info version 7.2.1.0 (CDC, Atlanta, GA, USA). The differences in the incidence of the PL and the absence of the isthmus between sexes were tested using a Pearson chi-square test. The mean of the parameters of the lobes of the thyroid gland were compared between the two sexes using independent samples t-test. A P-value less than 0.05 was considered as statistically significant.
The proposal was submitted to the Institutional Ethical Review Board of the University of Gondar for ethical consideration. The board approved the study is ethically acceptable and awarded the ethical clearance.
A total of 40 cadavers were observed and included in the study. Of those cadavers, 6 (15%) were female, and the rest 34 (85%) were male. About 55.0% of the study subjects (66.7% in female and 52.9% in male) show at least one among the anatomical variations and developmental anomalies of the thyroid gland. The right and left lobes of the thyroid gland were observed and present in all cadavers. The PL was noted in 52.5% (21/40) of the cadavers (Fig. 1). When the incidence of PL was compared between sexes, it was observed in 66.7% of female and 50% of male cadavers and it was not statically significant (P=0.451). In one of the male cadavers, the PL was found on both sides at the junctions of the isthmus to the lateral lobes. The length of right lobe measures 4 cm in 50% of the study subjects with a minimum of 3.00 cm and a maximum of 5.30 cm. The length of left lobe measures greater than 4 cm in 42.5% and its average width and thickness were 1.8 cm and 1.6 cm, respectively. The average length of the pyramidal lobes was 2.31 cm with a minimum of 0.5 cm and a maximum of 3.8 cm (Table 1). The mean length, width, and thickness of all lobes of the female cadavers were greater than the males. But, only the mean thickness of right lobe shows statically significant variation (P=0.021) between males and females (Table 2).
The origin of the PL was the junction of the isthmus to the left lobe in 57.1% of the cases. The apex of the PL was attached to the hyoid bone in 52.4% of the cases by LGT (Table 3, Figs. 2, 3). About 79.2% of the PLs were associated with LGT. The presence of LGT was significantly associated with the presence of PL (P<0.001).
In the present study, LGT was present in 40% of the cadavers. LGT was originated from the PL in all cases, but attached to the hyoid bone (hyopyramidalis) in 68.8% and to thyroid cartilage (thyreopyramidalis) in 31.25%.
An accessory thyroid tissue was found in one of the male cadavers (2.5%); it was found overlying the whole part of the isthmus and part of the underling tracheal ring. It was separated from the isthmus by the fascia covering it. This tissue was attached to the cricoid cartilage superiorly and tracheal rings inferiorly by a band of fasciae (Fig. 4).
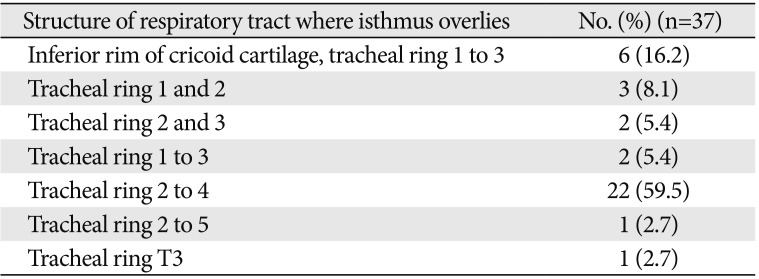
The isthmus of the thyroid gland was absent in 7.5% of the whole cadavers, 16.7% of female, and 5.9% of male cadavers (P=0.394) (Fig. 5). In 92.5% of the cadavers, it was found overlying the structure of the respiratory tract extending from the inferior rim of the cricoid cartilage to the fifth tracheal ring. In 59.5% of the individual, it overlies the 2nd to 4th tracheal rings. In one of the cadavers (2.5%), the isthmus was narrow and was only related to the 3rd tracheal ring (Table 4, Fig. 6).
An ectopic thyroid, thyroglossal cyst, and other possible developmental anomalies were searched in all cadavers in the embryonic pathway of the thyroid gland from the foramen cecum on the dorsum of the tongue to its definitive site in the neck. But, an ectopic thyroid and thyroglossal cyst were not detected.
The thyroid gland is a markedly labile gland that varies greatly in size and structure [2]. Most of the variable structures of the thyroid gland resulting from variable persistence of the embryonic thyroglossal duct [25]. Most of the diseases affecting the thyroid gland such as goiter, thyrotoxicosis, adenoma, carcinoma, etc. are usually associated with enlargement of the gland and require medical and surgical intervention [2].
Most of the anatomy textbooks stated that the lateral lobes of the thyroid gland measure a length of 5 cm; 3 cm width and 2 cm thickness [26]. In the current study, the average length of the right lobe was 4.24 cm and that of the left lobe was 4.08 cm. This finding explains the usual report that right lobe is slightly larger than the left one [711]. When compared to the findings of the other studies which investigated the size of the lateral lobes, this study was comparable to report of 4.32 cm length of the right and 4.22 cm to the left lobes by Joshi et al. [11], and 4.43 cm and 4.21 cm by Prakash et al. [24]. But, it was less than the report of 5.29 cm and 4.95 cm by Dixit et al. [27], and 5.32 cm and 4.74 cm by Asha et al. [5]. According to Prakash et al., the average width of the right lobe was 2.54 cm and it was 2.63 cm for that of the left lobe. The mean thickness of the right and left lobes was 1.13 cm and 1.18 cm, respectively as reported by Joshi et al. [11], 1.69 cm, and 1.7 cm as reported by Prakash et al. [24]. The mean thickness of both the right and left lobes was 1.6cm in the present study. The mean length, width, and thickness of all lobes of the female cadavers were greater than the male, which was similar to a study in Serbia [15] but in contrast to other studies [828].
The presence of PL could be a source of pitfalls in thyroid surgery, due to its frequency but unreliable preoperative diagnosis on scintigraphic images [1529]. In the present study, the PL was noted in 52.5% of the study subjects, which was closely related with the prevalence of the PL reported by most of the textbooks and cadaveric studies [13612252930]. But this finding was different from studies conducted by Asha et al. (0%) [5], Reddy and Panchakshari (21.7%) [6], Gaikwad and Joshi (26.59%) [3], Joshi et al. (37.77%) [11], Rajkonwar and Kusre (38.75%) [7], Muktyaz et al. (41.0%) [2], Ranade et al. (58.0%) [25], and Gurleyik et al. (65.7%) [31]. Although it was not statically significant, PL was observed more frequently in female than males, which was similar with the study by Zivic et al. [18], but in contrast to the study by Muktyaz et al. [2], Braun et al. [29], and Milojevic et al. [15].
The primary area for the origin of the PL in the present study was the junction of the left lobe with the isthmus. Milojevic et al. [15] reported the central part of the isthmus and junction of the right lobe with the isthmus as a primary area of origin to the PL.
According to the study of Joshi et al. [11], 47.05% of PL was attached to the left lobe and 32.55% to the right lobe. In the present study, only 4.8% of the PL originated from the left lobe or the right lobe. In the present study, a double PL was noted. This is a rare occurrence only reported in 3 cases reports and one cadaveric study [11173233].
In the present study, LGT was present in 40% of the cadavers. It was more frequently seen in female cadavers in contrast to the study of Muktyaz et al. [2]. The presence of LGT was 49.5% according to Ranade et al. [25], 30% according to Joshi et al. [11] and Kulkarni et al. [34], 30.85% as reported by Gaikwad and Joshi [3], 19.6% by Muktyaz et al. [2], and 18.25% by Rajkonwar et al. [7]. The LGT was primarily attached to the body of the hyoid bone in the present study which was similar to the study of Joshi et al. [11] and Rajkonwar and Kusre [7].
An accessory thyroid tissue is a rare developmental anomaly due to abnormal development of the thyroid gland. The accessory thyroid tissue could be asymptomatic or it could present with any disorder affecting the main thyroid gland including malignancy [35]. The occurrence of accessory thyroid tissue has been reported by different authors in literature and it was also seen in one of our cadavers [2325].
The absence of isthmus is risk factors for pathologic conditions like autonomous thyroid nodule, thyroiditis, primary carcinoma, neoplastic metastases, and infiltrative diseases [25]. In the present study, the isthmus of the thyroid gland was absent in 7.5% of the cadavers. Absence of isthmus was reported 10.0% by Kulkarni et al. [34], 12.5% by Muktyaz et al. [2], 14.6% by Dixit et al. [27], 16.66% by Joshi et al. [11], 20% by Asha et al. [5], 21.25% by Rajkonwar and Kusre [7], 29.78% by Gaikwad and Joshi [3], and 33% by Ranade et al. [25]. Like the other variations, the absence of isthmus was more frequently seen in female than male cadavers, which was comparable with a study of Gaikwad and Joshi [3] and Ranade et al. [25] but in contrast to the study by Muktyaz et al. [2] and Gaikawd and Joshi [3].
The relation of the isthmus to the tracheal rings was variable, found to be higher to the cricoid cartilage, and lower up to the 5th tracheal ring which was consistent with the finding of Joshi et al. [11]. In the current study, the isthmus was found mainly overlying the 2nd to 4th tracheal rings, which was consistent with the report of most of the anatomy textbooks and studies [1361236].
The main limitation of this study was the age of the cadavers was unknown and the variation of thyroid gland related to the age was not analyzed. The present study discovers the parameters of the lateral lobes of the thyroid gland, the prevalence of PL, absence of the isthmus, accessory thyroid tissue and LGT among Ethiopian cadavers that will hopefully increase information pole about the thyroid gland. This study was conducted on cadavers with no gross pathology. Further study is needed to compare anatomical variation and developmental anomalies between peoples affected by thyroid disorders and peoples not affected by thyroid disorders.
Acknowledgements
I have to thank a lot of people, including Mss Hiwot Ambaw, Mrs. Yezina Belay, and all others who were supporting and encouraging me during study design and data collection time. I also appreciate anatomy department staffs of all universities and private colleges where the study was conducted especially Mr. Abay Mulu, Mr. Kidanu Fekadu, Mr. Million Loha, and Mr. Girmay Tilahun for their unreserved support during the data collection time.
References
1. Thiagarajan B. Anatomy of thyroid gland surgeon's perspective. Otolaryngol Online J. 2015; 5:1–9.
2. Muktyaz H, Birendra Y, Dhiraj S, Arun SK. Anatomical variations of thyroid gland and its clinical significance in North Indian population. Global J Biol Agric Health Sci. 2013; 2:12–16.
3. Gaikwad S, Joshi R. An anatomical study of morphological variations of the thyroid gland. Int J Anat Res. 2016; 4:2665–2669.
4. Sinha MB, Siddiqui AU, Sharma DK. Levator glandulae thyroideae: a fibromusculoglandular band: a case report. Indian J Clin Anat Physiol. 2014; 1:57–59.
5. Asha S, Dixit D, Shirol VS, Bhimalli S. Study of absence of isthmus of thyroid gland with its developmental and surgical implications in adult human cadavers: a case series. Med Innov. 2013; 2:50–52.
6. Reddy CK, Panchakshari M. A study of anatomical and morphological variations of the thyroid gland. Sch J Appl Med Sci. 2016; 4:3510–3513.
7. Rajkonwar AJ, Kusre G. Morphological variations of the thyroid gland among the people of upper Assam region of northeast India: a cadaveric study. J Clin Diagn Res. 2016; 10:AC01–AAC3.
8. Şeker S, TaŞ İ. Determination of thyroid volume and its relation with isthmus thickness. Eur J Gen Med. 2010; 7:125–129.
9. Nikumbh RD, Nikumbh DB, Doshi MA. Multiple morphological variations in the thyroid gland: report of two cases. Int J Anat Res. 2015; 3:1476–1480.
10. Onwuaso IC, Samuel MN, Nwagbo ED. Age-related morphological changes in the foetal thyroid gland of white Fulani (Zebu) cattle. Indian J Anim Res. 2014; 48:438–443.
11. Joshi SD, Joshi SS, Daimi SR, Athavale SA. The thyroid gland and its variations: a cadaveric study. Folia Morphol (Warsz). 2010; 69:47–50. PMID: 20235050.
12. Moore K, Persaud TV, Torchia M. The developing human: clinical oriented embryology. 10th ed. Philadelphia, PA: Saunders;2015.
13. Chaudhary P, Singh Z, Khullar M, Arora K. Levator glandulae thyroideae, a fibromusculoglandular band with absence of pyramidal lobe and its innervation: a case report. J Clin Diagn Res. 2013; 7:1421–1424. PMID: 23998080.
14. Lokanadham S, Satheesh Naik K, Subhadra Devi V. Thyroid gland isthmus agenesis in autopsied fetus: case study. Prime Res Med. 2012; 2:69–71.
15. Milojevic B, Tosevski J, Milisavljevic M, Babic D, Malikovic A. Pyramidal lobe of the human thyroid gland: an anatomical study with clinical implications. Rom J Morphol Embryol. 2013; 54:285–289. PMID: 23771071.
16. Geraci G, Pisello F, Li Volsi F, Modica G, Sciumè C. The importance of pyramidal lobe in thyroid surgery. G Chir. 2008; 29:479–482. PMID: 19068184.
17. Hakeem AH, Hakeem IH, Wani FJ. Double pyramidal lobe of thyroid gland: a rare presentation. Thyroid Res Pract. 2016; 13:25–26.
18. Zivic R, Radovanovic D, Vekic B, Markovic I, Dzodic R, Zivaljevic V. Surgical anatomy of the pyramidal lobe and its significance in thyroid surgery. S Afr J Surg. 2011; 49:110. PMID: 21933507.
19. Sinos G, Sakorafas GH. Pyramidal lobe of the thyroid: anatomical considerations of importance in thyroid cancer surgery. Oncol Res Treat. 2015; 38:309–310. PMID: 26045028.
20. Kotisso B, Ersumo T, Ali A, Wassie A. Thyroid disease in Tikur Anbessa Hospital: a five-year review. Ethiop Med J. 2004; 42:205–209. PMID: 16895039.
21. Debebe K, Genetu G, Feleke Y, Kebede T. Pattern, clinical presentation and pregnancy outcome of thyroid diseases in pregnant women at national endocrine referral clinic of Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia from june 2010 to june 2015. J Thyroid Disord Ther. 2017; 6:209.
22. Abuye C, Berhane Y, Akalu G, Getahun Z, Ersumo T. Prevalence of goiter in children 6 to 12 years of age in Ethiopia. Food Nutr Bull. 2007; 28:391–398. PMID: 18274165.
23. Won SY. Anatomical considerations of the superior thyroid artery: its origins, variations, and position relative to the hyoid bone and thyroid cartilage. Anat Cell Biol. 2016; 49:138–142. PMID: 27382516.
24. Prakash , Rajini T, Ramachandran A, Savalgi GB, Venkata SP, Mokhasi V. Variations in the anatomy of the thyroid gland: clinical implications of a cadaver study. Anat Sci Int. 2012; 87:45–49. PMID: 21956789.
25. Ranade AV, Rai R, Pai MM, Nayak SR, Prakash , Krisnamurthy A, Narayana S. Anatomical variations of the thyroid gland: possible surgical implications. Singapore Med J. 2008; 49:831–834. PMID: 18946620.
26. Standring S. Gray's anatomy: the anatomical basis of clinical practice. London: Elsevier Churchill Livingstone;2005.
27. Dixit D, Shilpa MB, Harsh MP, Ravishankar MV. Agenesis of isthmus of thyroid gland in adult human cadavers: a case series. Cases J. 2009; 2:6640. PMID: 20181171.
28. Rashid SQ. Thyroid gland standard for Bangladeshi population and prevalence of unknown pathologies in the normal population. J Med Ultrasound. 2016; 24:101–106.
29. Braun EM, Windisch G, Wolf G, Hausleitner L, Anderhuber F. The pyramidal lobe: clinical anatomy and its importance in thyroid surgery. Surg Radiol Anat. 2007; 29:21–27. PMID: 17146601.
30. Moore KL, Dalley AF. Clinically oriented anatomy. 5th ed. Toronto: Lippincott Williams & Wilkins;2006.
31. Gurleyik E, Gurleyik G, Dogan S, Cobek U, Cetin F, Onsal U. Pyramidal lobe of the thyroid gland: surgical anatomy in patients undergoing total thyroidectomy. Anat Res Int. 2015; 2015:384148. PMID: 26236507.
32. Kaklamanos I, Zarokosta M, Flessas I, Zoulamoglou M, Katsoulas T, Birbas K, Troupis T, Mariolis-Sapsakos T. Surgical anatomy of double pyramidal lobe on total thyroidectomy: a rare case report. J Surg Case Rep. 2017; 2017:rjx035. PMID: 28458845.
33. Ignjatović M. Double pyramidal thyroid lobe. J Postgrad Med. 2009; 55:41–42. PMID: 19242079.
34. Kulkarni V, Sreepadma S, Deshpande SK. Morphological variations ot the thyroid gland. Med Innov. 2012; 1:35–38.
35. Sawant DA, Moore TF. An unusual course of segmental renal artery displays a rare case of hilar nutcracker phenomenon. Case Rep Med. 2015; 2015:249015. PMID: 26448765.
36. Snell RS. Clinical anatomy by regions. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2008.
Fig. 1
Pyramidal lobe originating from the central part of the isthmus and its apex attached to hyoid bone by levator glandulae thyroidae (LGT) in male cadaver. CCA, common carotid artery; IJV, internal jugular vein.
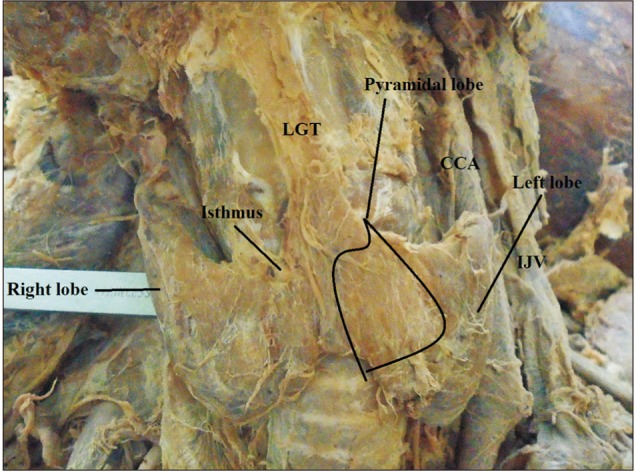
Fig. 2
Pyramidal lobe originating from the junction of the isthmus to the left lobe with levator glandulae thyroidae (LGT) attached to the hyoid bone.
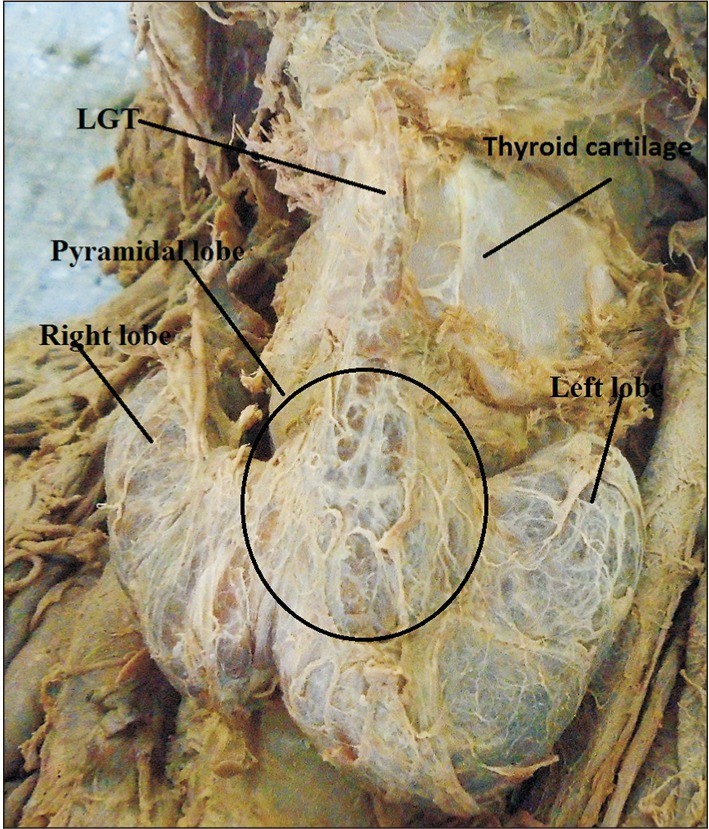
Fig. 4
An accessory thyroid tissue overlying the isthmus along with right and left lobes. CCA, common carotid artery; IJV, internal jugular vein.
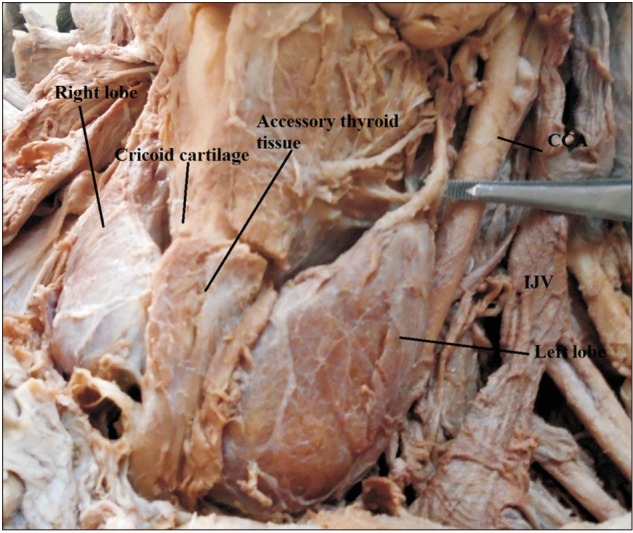
Fig. 5
The isthmus is absent; the pyramidal lobe originates from the left lobe and attached to the hyoid bone in a female cadaver. LGT, levator glandulae thyroidae.
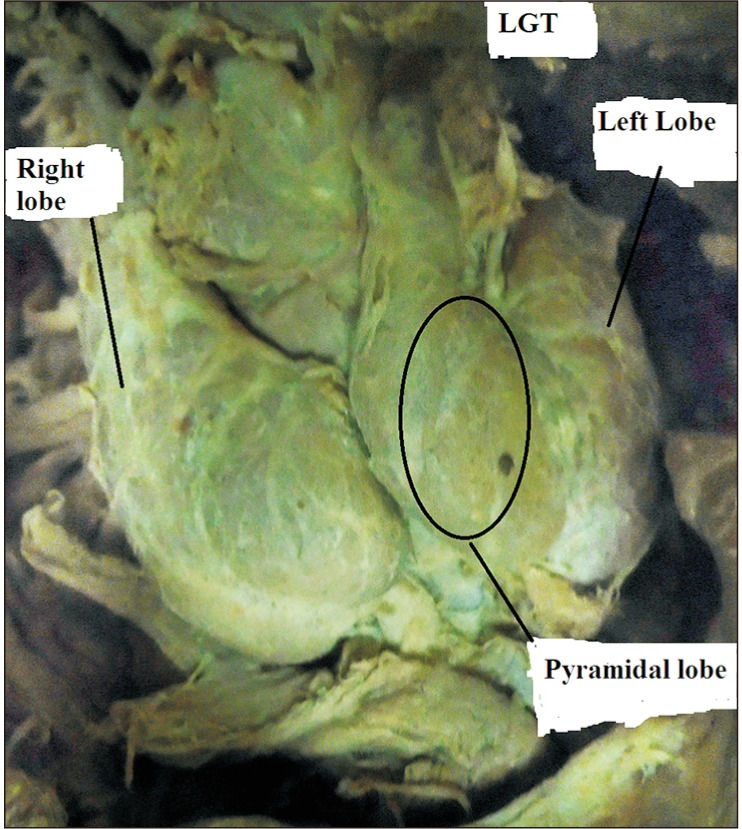
Fig. 6
The right and left lobes of the thyroid gland with a narrow isthmus related to the 3rd tracheal ring.
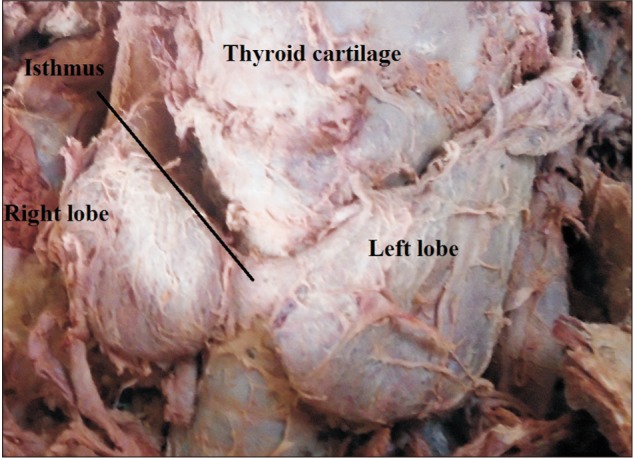
Table 1
Details of the length, width, and thickness of the lobes of the thyroid gland
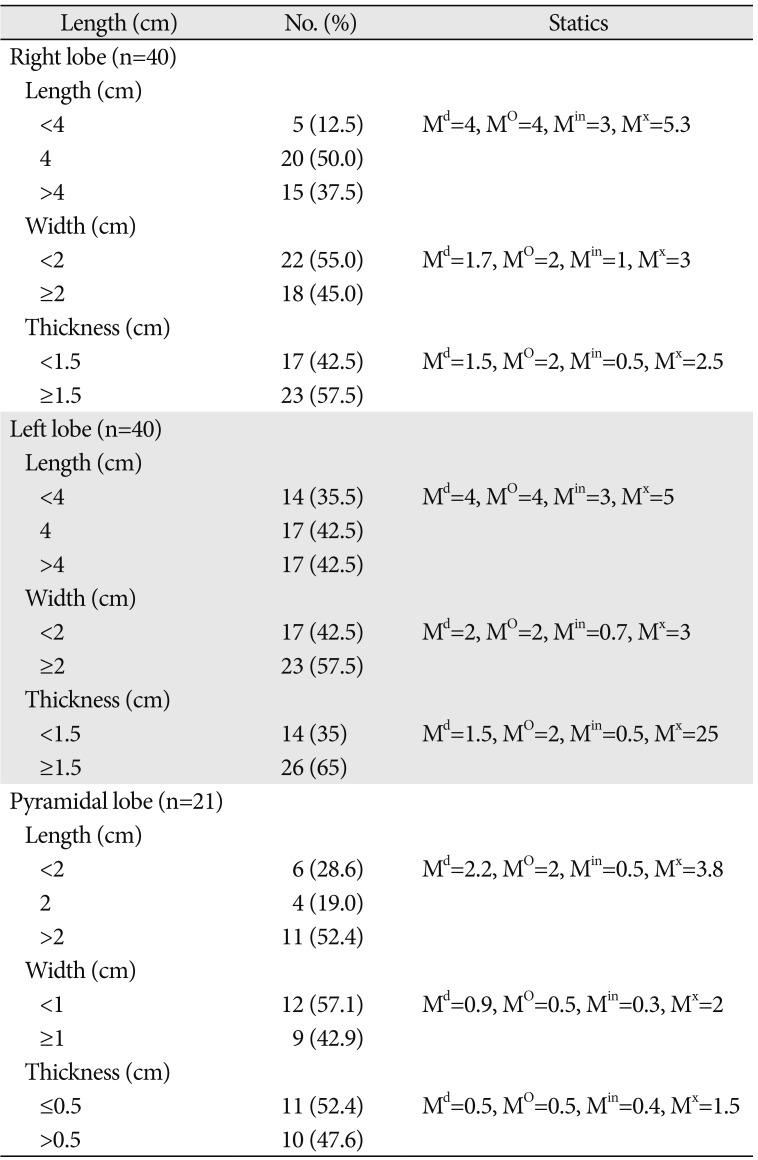
Table 2
Comparisons of the parameters of the thyroid glands between male and female by centimeter
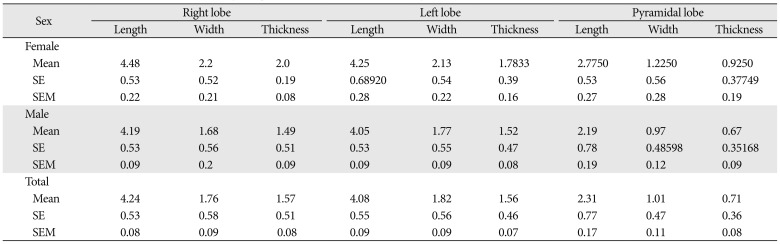
Table 3
The origin of the pyramidal lobe and its attachment by levator glandulae thyroidae
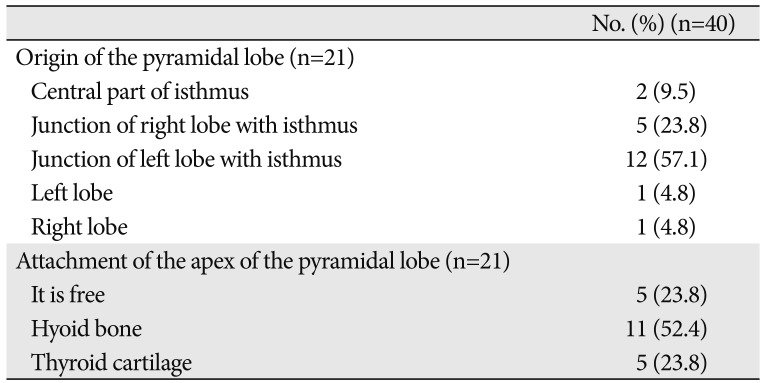
Table 4
Relation of the isthmus of the thyroid gland to the structure of respiratory tract




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download