Abstract
Allergic diseases are a significant health concern in developing countries. Type-A procyanidin polyphenols from cinnamon (Cinnamomum zeylanicum Blume) bark (TAPP-CZ) possesses antiasthmatic and antiallergic potential. The present study was aimed at the possible anti-allergic mechanism of TAPP-CZ against the compound 48/80 (C48/80)–induced mast cell degranulation in isolated rat peritoneal mast cells (RPMCs). TAPP-CZ (1, 3, 10, and 30 µg/ml) was incubated for 3 hours with isolated, purified RPMCs. The C48/80 (1 µg/ml) was used to induce mast cell degranulation. The mast cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay whereas histamine, β-hexosaminidase (β-HEX), and interleukin-4 (IL-4) levels were determined in RPMCs. TAPP-CZ (3, 10, and 30 µg/ml) showed significant and dose-dependent decrease in a number of degranulated cells and levels of markers (histamine, β-HEX, and IL-4) as compared with C48/80 control. In conclusion, TAPP-CZ stabilizes mast cell and cause inhibition of the allergic markers such as histamine, IL-4, and β-HEX in IgE-mediated manner. The present study supports mast cell stabilization as a possible mechanism of action of TAPP-CZ against immune respiratory disorders such as asthma and allergic rhinitis.
Allergic diseases of the respiratory system such as asthma and rhinitis are a significant health concern in developing countries due to increased environmental pollution [1]. The rapid growth in the prevalence, severity, and complexity of allergic respiratory diseases in modern population imposes a significant burden on quality of life and health-care costs [2]. The close association between mast cell and early-phase of hypersensitivity plays a vital role in allergic diseases [34]. The specific antigen such as FcεRI activates the mast cells that are present in all organs and tissues [56]. The IgE/FcεRI-mediated activation causes release of various chemical mediators in early-phase (such as histamine, β-hexosaminidase [β-HEX], proteoglycans, proteases, and tryptase) as well as in late-phase (proinflammatory cytokines such as tumor necrosis factor α [TNF-α], interleukin [IL]-1β, IL-4, IL-5, IL-6, IL-13, transforming growth factor-β, and vascular endothelial growth factor). These mediators play an important role in induction and maintenance of immune-inflammatory reactions [7].
An array of studies over past decade confirmed compound 48/80 (C48/80) as a potent and well-established agent for mast cell degranulation and to evaluate the mechanism of new therapeutic moieties against allergic reactions [89]. C48/80 can rapidly release inflammatory substances including histamine from rat peritoneal mast cells (RPMCs) [10]. This allergic inflammatory influx results in various pathophysiologic events including vasodilation, increase vascular permeability, tissue edema, bronchial and intestinal smooth muscle contraction, and elevated mucus production [11]. These conditions contributes to various disease state including allergic hyper-responsiveness, migraine [12], pain [1314], interstitial cystitis [15], irritable bowel syndrome [16], and endometriosis [17].
Over past decade, disodium cromoglycate (DSCG) has been used extensively as a “mast cell stabilizer” and serves as a treatment option for management of various allergic diseases including asthma, allergic rhinitis, allergic conjunctivitis, and mastocytosis [1819]. However, use of DSCG for the mast cell stabilization is limited because of its nephrotoxicity [1920]. Therefore, search for novel agents derived from plant material for mast cell stabilization potential is ongoing. Researchers reported therapeutic potential of many isolated moieties from herbal extracts such as curcumin [9], caffeic acid [21], resveratrol [22], and epigallocatechin gallate [23] against mast cell degranulation. Furthermore, polyphenols isolated from apple extract was reported for the inhibitory potential against mast cell degranulation via inhibition of binding between FcεRI and IgE [242526].
Type-A proanthocyanidins polyphenols isolated from cinnamon (Cinnamomum zeylanicum Blume, Family: Lauraceae) bark (TAPP-CZ) shown to have an array of therapeutic applications for the treatment of immune inflammation, arthritis, asthma, and viral infection [2728293031]. Recently, the anti-allergic potential of TAPP-CZ against ovalbumin-induced experimental allergic rhinitis has been reported in mice after its intranasal administration [32]. However, the molecular mechanism(s) of action of TAPP-CZ for the management of allergic responses remains unresolved. Hence, the present study aimed at the mast cell stabilization as a possible mechanism for the anti-allergic action of TAPP-CZ against the mast cell degranulation induced by C48/80 on isolated RPMCs.
Sprague-Dawley rats (180–200 g) were purchased from National Toxicology Centre, Pune and kept in quarantine for 1 week. Experimental protocol (SIOP/IAEC/2011/22) was approved by the Institutional Animal Ethics Committee of Sinhgad Institute of Pharmacy, Pune, India. The rats were maitained in the animal house of the institute under standard laboratory conditions (temperature of 24±1℃, relative humidity of 45%–55% and 12:12 hour light/dark cycle) throught the study. Animals had free access to standard chow pelleted food (Pranav Agro-industries Ltd., Sangli, India) and water ad libitum. Animals were brought to the testing laboratory 1 hour for adaptation purpose before the experiments timings (between 10:00 AM to 5:00 PM).
C48/80, MTT, and Percoll were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DSCG was purchased from Ahlcon Parenterals (India) Ltd. (New Delhi, India). All other reagents were purchased from SD Fine Chemicals, Mumbai, India. Enzyme-linked immunosorbent assay (ELISA) kits for histamine (Cat No. BA D-0024-10499 from Labor Diagnostika Nord GmbH & Co. KG, Nordhorn, Germany), IL-4 (Cat No. ELR-IL4-001C from RayBiotech Inc., Norcross, GA, USA), and β-HEX (Cat No. E90637Ra from USCN Life Science Inc., GmbH, Germany) were purchased from respective suppliers.
The test compound, TAPP-CZ, prepared as per reported procedure [3233], was provided by Indus Biotech Private Limited (Pune, India). TAPP-CZ was characterized by high-performance liquid chromatography fingerprinting (Jasco, Tokyo, Japan) and found to match with characteristics of TAPP-CZ from cinnamon bark as reported earlier [3435]. The TAPP-CZ contains pentameric type-A procyanidins flavonoid (75.9% purity) as a marker compound with dimer (1.7%), trimer (11.1%), and tetramers (2.266%). The test compound complies with quality requirements related to the absence of microbial content and heavy metals.
The RPMCs were isolated as per previously reported procedure [36]. In brief, rats were anesthetized with ether, injected with 10 ml of calcium-free hydroxyethyl piperazineethanesulfonic acid (HEPES)-Tyrode buffer into the peritoneal cavity, and the abdomen gently massaged for approximately 90 seconds. The peritoneal cavity was opened and the fluid aspirated using a Pasteur pipette. RPMCs were purified using a Percoll density gradient as per reported procedure [37]. RPMC preparation was approximately 95% pure as assessed by toluidine blue staining [38]. Purified RPMCs (1×106 cells/ml) were resuspended in HEPES-Tyrode buffer.
The viability of RPMCs was tested by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay as per reported method [39]. Briefly, RPMCs (2×105 cells/well) were pretreated with various concentrations of either vehicle (distilled water, 10 µg/ml), DSCG (10 and 100 µg/ml), or TAPP-CZ (1, 3, 10, and 30 µg/ml) at 37℃ for 3 hours. After addition of MTT (5 mg/ml saline), RPMCs was incubated at 37℃ for 4 hours. After washing out the supernatant, the insoluble formazan the crystallized MTT was dissolved in dimethyl sulfoxide and the absorbance was measured at 570 nm with a spectrophotometer (UV-visible spectrophotometer, V-630, Jasco). The density of formazan formed by vehicle RPMCs was taken as 100% of viability.
Cell viability was checked by a trypan blue dye (0.4%) exclusion test [38]. RPMCs (2×105 cells/well) were pretreated with various concentrations of either DSCG (10 and 100 µg/ml) or TAPP-CZ (1, 3, 10, and 30 µg/ml) or vehicle (10 µg/ml) at 37℃ for 3 hours and was incubated with C48/80 (1 µg/ml) at 37℃ for 10 minutes. The mast cell numbers (total and degranulated) were counted under high power microscope (Motic digital microscope, Motic Asia-Pacific, Hong Kong) for a set of 100 cells per treatment group. The % intact cells were calculated by the formula: Percentage of intact mast cells=(Total number of mast cells−Total number of degranulated cells)/(Total number of mast cells)×100.
The RPMCs suspensions (2×105 cells/ml) were pretreated with various concentrations of either DSCG (10 and 100 µg/ml) or TAPP-CZ (1, 3, 10, and 30 µg/ml) or vehicle (10 µg/ml) at 37℃ for 3 hours and incubated with C48/80 (0.25 µg/ml) for 15 minutes. The cells were separated from the released histamine by centrifugation (at 400 ×g for 5 minutes at 4℃). Residual histamine in the cells was released by disrupting the cells with perchloric acid and centrifugation at 400 ×g for 5 minutes at 4℃. The histamine content was measured by using histamine ELISA assay kit on a microplate reader at 450 nm.
The β-HEX assay was performed as described previously with some modifications [40]. The RPMCs suspensions (2×105 cells/ml) were pretreated with various concentrations of either DSCG (10 and 100 µg/ml) or TAPP-CZ (1, 3, 10, and 30 µg/ml) or vehicle (10 µg/ml) at 37℃ for 3 hours and incubated with C48/80 (0.25 µg/ml) for 15 minutes. The 100 µl of supernatant was used for the β-HEX enzyme ELISA. Briefly, 100 µl of supernatant was added to β-HEX substrate buffer (100 mM sodium citrate, 1 mM 4-nitrophenyl N-acetyl-β-D-galactosaminide, pH 4.5) and incubated for 1 hour at 37℃. The reaction was terminated using stop solution, and absorbance was measured using a microplate reader at 450 nm.
The RPMCs suspensions (2×105 cells/ml) were pretreated with various concentrations of either DSCG (10 and 100 µg/ml) or TAPP-CZ (1, 3, 10, and 30 µg/ml) or vehicle (10 µg/ml) at 37℃ for 3 hours and incubated with C48/80 (0.25 µg/ml) for 15 minutes. The 100 µl of supernatant was used for IL-4 sandwich ELISA as per manufacturer's instructions (RayBiotech, Inc.) using a microplate reader at 450 nm.
The data was presented as a mean±standard error of mean (SEM) and analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA). Dunnett's test was applied for post hoc analysis. The P-values less than 0.05 (two-sided) were considered significant.
The cell viability remains 100% in TAPP-CZ (3 and 30 µg/ml) and DSCG (100 µg/ml) treated RPMCs during MTT assay. The % viability of the RPMCs after pretreatment with TAPP-CZ (1 and 10 µg/ml) as well as DSCG (10 µg/ml) was found to be around 80% (Table 1).
The C48/80 treated control RPMCs showed significant (P<0.001) increase in the number of degranulated cell and decrease in intact cells as compared to normal RPMCs. The DSCG pretreated (10 and 100 µg/ml) and TAPP-CZ pretreated (3, 10, and 30 µg/ml) RPMCs showed significant (P<0.001) reduction in the number of degranulated cells as compared with C48/80-treated control RPMCs (Table 1).
Normal RPMCs were oval and contained many fine granules surrounding a prominent nucleus (Fig. 1A). After stimulation with C48/80, RPMCs were degranulated (characterized by cell swelling) and showed the presence of extruded granules near the cell surface (Fig. 1B). When RPMCs were pretreated with DSCG (100 µg/ml) and TAPP-CZ (3, 10, and 30 µg/ml), they showed inhibition of C48/80-induced degranulation (Fig. 1C–F, respectively).
A significant increase (P<0.001) in the level of histamine in C48/80-treated control RPMCs were found as compared to normal RPMCs. Pretreatment with TAPP-CZ (10 and 30 µg/ml) showed the significant attenuation (P<0.05 and P<0.001, respectively) of elevated histamine levels as compared to C48/80-treated control RPMCs. Pretreatment with DSCG (10 and 100 µg/ml) showed significant decrease (P<0.001) in histamine the levels as compared to C48/80-treated control RPMCs (Fig. 2A). The half maximal inhibitory concentration (IC50) of TAPP-CZ for histamine release from RPMCs was found to be 1.48 µg/ml.
The β-HEX levels of C48/80-treated control RPMCs were significantly (P<0.001) more than normal RPMCs. TAPP-CZ (10 and 30 µg/ml) treated RPMCs showed significant and dose dependant attenuation (P<0.05 and P<0.001) in β-HEX levels as compared to C48/80-treated control RPMCs. DSCG (10 and 100 µg/ml)-treated RPMCs also showed the significant (P<0.001) decrease in the elevated levels of β-HEX levels as compared to C48/80-treated control RPMCs (Fig. 2B).
A significant increase (P<0.001) in the level of IL-4 was found in C48/80-treated control RPMCs as compared to normal RPMCs. The significant and dose-dependent attenuation (P<0.01 and P<0.001) of IL-4 level was found in TAPP-CZ (10 and 30 µg/ml)-treated RPMCs as compared to C48/80-treated control RPMCs. The elevated level of IL-4 was significantly (P<0.001) inhibited by the pretreatment of DSCG (10 and 100 µg/ml) when compared with control RPMCs (Fig. 2C). The IC50 of TAPP-CZ for IL-4 release from RPMCs was found to be 24.44 µg/ml.
Mast cell plays a significant role in acute and chronic allergic inflammation after FcεRI stimulation by releasing various proinflammatory mediators such as histamine, cytokines, chemokines, and leukotrienes [4142]. Allergy is known as type I hypersensitive or early-phase of reaction to various allergens occurred in response to activation of mast cells and basophils via interaction with IgE, resulting in immune-inflammatory responses [114143]. The mast cell contains high-affinity receptor, FcεRI, on its surface with an ability of antigen-specific IgE binding [4144]. C48/80 is the condensed product of N-methoxy phenylamine with formaldehyde, which is the most potent mast cell degranulator amongst the various secretagogues [45]. C48/80 has an ability to induce membrane perturbation that increases the permeability of the lipid bilayer membrane [46]. Moreover, its interaction with mast cell caused the release of almost 90% of histamine as compared to natural process. Therefore, in the present investigation, we used C48/80 to evaluate the mechanisms of action of TAPP-CZ against RPMCs. Furthermore, pre-treatment of RPMCs with TAPP-CZ retains its normal morphology, showed significant inhibition in a number of degranulated cell and histamine, IL-4 as well as β-HEX release from RPMCs.
In allergic patients, allergen challenge causes an early-phase reaction within an hour which is followed by a late-phase reaction after 3 to 48 hours [47]. A compelling body of evidence supports that the early-phase reaction which is an acute inflammatory process is characterized by the release of histamine and other pharmacological mediators from mast cells [4849]. Whereas, the release of pro-inflammatory cytokines such as TNF-α and IL's in various tissues including lung, nose, skin, and retina is a hallmark for the late phase reaction which is the clinical manifestation of chronic allergic diseases [5051]. Mast cells are involved in both early and late phase reaction in IgE-mediated allergic reactions.
The mast cell serves as a storehouse for histamine which is synthesis from its precursor L-histidine via activation of enzyme histidine decarboxylase. The release of histamine from activated mast cell results in proinflammatory response as well as modulation of the activity of a variety of cells including T cells, monocytes, macrophages, neutrophils and eosinophils [52535455]. These mediators are responsible for induction and maintenance of acute inflammation and early-phase of hypersensitivity [56]. Thus, mast cells played a central dogma role in early-type hypersensitivity and allergic diseases. In the present investigation, RPMCs stimulated with C48/80 resulted in mast cell degranulation thus elevates the levels of histamine whereas pretreatment with TAPP-CZ attenuated the elevated levels of histamine which might be due to inhibition of mast cells degranulation. Results of the present investigation are in line with the findings of the previous investigator where polyphenol treatment significantly inhibited histamine release from RPMCs [25].
The enzyme β-HEX is considered as the major mediator of the acute inflammation and early hypersensitivity responses along with histamine. It has been reported that interaction of β-HEX with cell-surface receptors resulted in the development of various symptoms such as acute rhinitis and bronchoconstriction [57]. Thus, controlling the release of histamine and β-HEX from mast cells is thought to be important targets for attenuation of various allergic conditions [4158]. Interestingly, in the present study, we found a similar result where incubation of C48/80 with RPMCs induced the release of β-HEX whereas pretreatment with TAPP-CZ showed inhibition of elevated β-HEX levels.
Recently, some researchers have reported that late-phase reaction is more accurate clinical features of disease as compared to early-phase response, as it involved pro-inflammatory mediators along with various types of cells [259]. During the late-phase reaction, the eosinophils are recruited after IgE-mediated stimulation of mast cell degranulation [60]. IL-4 is reported to act as an eosinophil chemoattractant that enables endothelial cells to produce eosinophil chemotactic factor and eotaxin [61]. It also plays an important role in conversion of naïve T cells to allergic Th2 cells. Thus, IL-4-dependent recruitment of eosinophil responsible for elevated inflammation during allergic diseases [62]. Clinically, it has been shown that patients with allergic rhinitis are associated with high serum IL-4 levels as compared to healthy controls [63]. These findings depicted the importance of inhibition of pro-inflammatory cytokines release from mast cell in amelioration of allergic inflammation. Our results of mast cell degranulation by C48/80 with elevated IL-4 levels are in agreement with the existing literature [6264]. However, pretreatment with TAPP-CZ inhibits mast cell degranulation and subsequently decreases the level of IL-4 to induce mast-cell-mediated allergic reactions.
Research revealed that allergic reactions could be significantly suppressed through attenuating the interaction between IgE and FcεRI [6566]. Numerous scientific studies also demonstrated that the compound with an ability to inhibit the binding of IgE antibody and FcεRI possesses strong anti-allergic action [8924]. The procyanidin polyphenol-enriched apple extract is reported to inhibit mast cell degranulation in-vitro via decreasing the intracellular cross-linking of IgE and FcεRI thus suggesting its antiallergenic potential [2467]. Furthermore, piceatannol, a stilbene-type polyphenol, was shown to inhibit mast cell degranulation and secretion of various mediators via decreasing IgE and FcεRI interaction [6869]. Another naturally occurring polyphenol compound, chlorogenic acid, was shown to inhibit mast cell-dependent anaphylactic reaction [70]. In the past, anti-allergic effects of TAPP-CZ against ovalbumin-induced allergic rhinitis in mice was shown to be mediated by serum IgE level inhibition [32]. Therefore, mast cell stabilization activity of TAPP-CZ can be attributed, at least partly, to the IgE inhibition.
In conclusion, the TAPP-CZ obtained from cinnamon bark demonstrated the beneficial effect against C48/80 induced of mast cell degranulation, probably due to inhibition of the cross-linking of IgE with FcεRI and reduces the levels of the allergic makers such as histamine, β-HEX, and IL-4.
Acknowledgements
The authors would like to acknowledge Dr. K. G. Bothara, Principal, Sinhgad Institute of Pharmacy, Narhe, Pune, India, for infrastructural facilities. The study was supported by Indus Biotech Private Limited, Pune, India.
References
1. Takizawa H. Impact of air pollution on allergic diseases. Korean J Intern Med. 2011; 26:262–273. PMID: 22016586.
2. Kay AB. Overview of ‘allergy and allergic diseases: with a view to the future’. Br Med Bull. 2000; 56:843–864. PMID: 11359624.
3. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005; 23:749–786. PMID: 15771585.
4. Puxeddu I, Piliponsky AM, Bachelet I, Levi-Schaffer F. Mast cells in allergy and beyond. Int J Biochem Cell Biol. 2003; 35:1601–1607. PMID: 12962699.
5. Pearce FL, Befus AD, Gauldie J, Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982; 128:2481–2486. PMID: 6176639.
6. Murrant T, Bihari D. Anaphylaxis and anaphylactoid reactions. Int J Clin Pract. 2000; 54:322–328. PMID: 10954960.
7. Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009; 21:666–678. PMID: 19828304.
8. Choi YH, Chai OH, Han EH, Choi SY, Kim HT, Song CH. Lipoic acid suppresses compound 48/80-induced anaphylaxis-like reaction. Anat Cell Biol. 2010; 43:317–324. PMID: 21267406.
9. Choi YH, Yan GH, Chai OH, Song CH. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat Cell Biol. 2010; 43:36–43. PMID: 21190003.
10. Nishikawa H, Kitani S. Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol. 2008; 8:1207–1215. PMID: 18602066.
11. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008; 454:445–454. PMID: 18650915.
12. Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007; 130:166–176. PMID: 17459586.
13. Oliveira SM, Drewes CC, Silva CR, Trevisan G, Boschen SL, Moreira CG, de Almeida Cabrini D, Da Cunha C, Ferreira J. Involvement of mast cells in a mouse model of postoperative pain. Eur J Pharmacol. 2011; 672:88–95. PMID: 22004612.
14. Drummond PD. The effect of cutaneous mast cell degranulation on sensitivity to heat. Inflamm Res. 2004; 53:309–315. PMID: 15241566.
15. Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One. 2008; 3:e2096. PMID: 18461160.
16. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004; 126:693–702. PMID: 14988823.
17. Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noël JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006; 86:1336–1343. PMID: 17007852.
18. Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006; 1088:78–99. PMID: 17192558.
19. Miyatake A, Fujita M, Nagasaka Y, Fujita K, Tamari M, Watanabe D, Nakano N, Hidari KI, Suzuki Y. The new role of disodium cromoglycate in the treatment of adults with bronchial asthma. Allergol Int. 2007; 56:231–239. PMID: 17519581.
20. Beach JE, Blair AM, Clarke AJ, Bonfield CT. Cromolyn sodium toxicity studies in primates. Toxicol Appl Pharmacol. 1981; 57:367–400. PMID: 6784269.
21. Hossen MA, Inoue T, Shinmei Y, Minami K, Fujii Y, Kamei C. Caffeic acid inhibits compound 48/80-induced allergic symptoms in mice. Biol Pharm Bull. 2006; 29:64–66. PMID: 16394511.
22. Han SY, Bae JY, Park SH, Kim YH, Park JH, Kang YH. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J Nutr. 2013; 143:632–639. PMID: 23514766.
23. Li GZ, Chai OH, Song CH. Inhibitory effects of epigallocatechin gallate on compound 48/80-induced mast cell activation and passive cutaneous anaphylaxis. Exp Mol Med. 2005; 37:290–296. PMID: 16155406.
24. Tokura T, Nakano N, Ito T, Matsuda H, Nagasako-Akazome Y, Kanda T, Ikeda M, Okumura K, Ogawa H, Nishiyama C. Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between IgE and FcεRI. Biosci Biotechnol Biochem. 2005; 69:1974–1977. PMID: 16244451.
25. Kanda T, Akiyama H, Yanagida A, Tanabe M, Goda Y, Toyoda M, Teshima R, Saito Y. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci Biotechnol Biochem. 1998; 62:1284–1289. PMID: 9720210.
26. Matsuo N, Yamada K, Shoji K, Mori M, Sugano M. Effect of tea polyphenols on histamine release from rat basophilic leukemia (RBL-2H3) cells: the structure-inhibitory activity relationship. Allergy. 1997; 52:58–64. PMID: 9062630.
27. Ravindran PN, Nirmal Babu K, Shylaja M. Cinnamon and cassia: the genus Cinnamomum. Boca Raton, FL: CRC Press;2004.
28. Cao H, Anderson RA. Cinnamon polyphenol extract regulates tristetraprolin and related gene expression in mouse adipocytes. J Agric Food Chem. 2011; 59:2739–2744. PMID: 21329350.
29. Joshi SS, Kuszynski CA, Bagchi M, Bagchi D. Chemopreventive effects of grape seed proanthocyanidin extract on Chang liver cells. Toxicology. 2000; 155:83–90. PMID: 11154800.
30. Vetal S, Bodhankar SL, Mohan V, Thakurdesai PA. Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Sci Hum Wellness. 2013; 2:59–67.
31. Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA. Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol. 2013; 3:23–30.
32. Aswar UM, Kandhare AD, Mohan V, Thakurdesai PA. Anti-allergic effect of intranasal administration of type-A procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized BALB/c mice. Phytother Res. 2015; 29:423–433. PMID: 25504814.
33. Kandhare A, Aswar U, Mohan V, Bodhankar SL, Thakudesai PA. Effect of Type-A procyanidine polyphenols from bark of Cinnamomum Zeylanicum on allergic rhinitis model in balb-c mice [GU-6]. In : 45th Annual Conference of Indian Pharmacological Society (IPSCON-2012) and International Conference on “Navigating Pharmacology towards Safe and Effective Therapy”; 2013 Jan 5-7; Nagpur, India. Hyderabad: Indian Pharmacological Society;2013.
34. Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, Schoene NW, Graves DJ. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004; 52:65–70. PMID: 14709014.
35. Lazarus SA, Adamson GE, Hammerstone JF, Schmitz HH. High-performance liquid Chromatography/Mass spectrometry analysis of proanthocyanidins in foods and beverages. J Agric Food Chem. 1999; 47:3693–3701. PMID: 10552707.
36. Cochrane DE, Douglas WW. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974; 71:408–412. PMID: 4205591.
37. Hachisuka H, Nomura H, Sakamoto F, Mori O, Okubo K, Sasai Y. Effect of antianaphylactic agents on substance-P induced histamine release from rat peritoneal mast cells. Arch Dermatol Res. 1988; 280:158–162. PMID: 2454082.
38. Mascotti K, McCullough J, Burger SR. HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion. 2000; 40:693–696. PMID: 10864990.
39. Yoshimura T, Hamaguchi E, Usami E, Nakashima K, Kawaguchi M, Suzuki N, Okamoto Y, Nakao T, Yamazaki F. Increased in vitro release of interferon-gamma from ampicillin-stimulated peripheral blood mononuclear cells in Stevens-Johnson syndrome. Biol Pharm Bull. 2004; 27:929–931. PMID: 15187450.
40. Gao Y, Hou R, Fei Q, Fang L, Han Y, Cai R, Peng C, Qi Y. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci Rep. 2017; 7:38736. PMID: 28045016.
41. Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev. 2011; 242:144–160. PMID: 21682743.
42. Aung G, Niyonsaba F, Ushio H, Kajiwara N, Saito H, Ikeda S, Ogawa H, Okumura K. Catestatin, a neuroendocrine antimicrobial peptide, induces human mast cell migration, degranulation and production of cytokines and chemokines. Immunology. 2011; 132:527–539. PMID: 21214543.
43. Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011; 66:725–732. PMID: 21466562.
44. Sada K, Miah SM, Maeno K, Kyo S, Qu X, Yamamura H. Regulation of FcεRI-mediated degranulation by an adaptor protein 3BP2 in rat basophilic leukemia RBL-2H3 cells. Blood. 2002; 100:2138–2144. PMID: 12200378.
45. Theoharides TC, Kops SK, Bondy PK, Askenase PW. Differential release of serotonin without comparable histamine under diverse conditions in the rat mast cell. Biochem Pharmacol. 1985; 34:1389–1398. PMID: 2581583.
46. Tasaka K, Mio M, Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986; 56:464–469. PMID: 2424349.
47. Gleich GJ. The late phase of the immunoglobulin E-mediated reaction: a link between anaphylaxis and common allergic disease? J Allergy Clin Immunol. 1982; 70:160–169. PMID: 6125535.
48. Lewis RA, Robin JL, Austen KF. Pharmacologic regulation of mediator generation and release from the murine bone marrow derived mast cell. Int Arch Allergy Appl Immunol. 1985; 77:121–125. PMID: 2409010.
49. Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990; 62:5–33. PMID: 2404155.
50. Charlesworth EN, Hood AF, Soter NA, Kagey-Sobotka A, Norman PS, Lichtenstein LM. Cutaneous late-phase response to allergen: mediator release and inflammatory cell infiltration. J Clin Invest. 1989; 83:1519–1526. PMID: 2468688.
51. Kalinen M. Hypotheses on the contribution of late-phase allergic responses to the understanding and treatment of allergic diseases. J Allergy Clin Immunol. 1984; 73:311–315. PMID: 6366029.
52. Jutel M, Klunker S, Akdis M, Malolepszy J, Thomet OA, Zak-Nejmark T, Blaser K, Akdis CA. Histamine upregulates Th1 and downregulates Th2 responses due to different patterns of surface histamine 1 and 2 receptor expression. Int Arch Allergy Immunol. 2001; 124:190–192. PMID: 11306965.
53. Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001; 413:420–425. PMID: 11574888.
54. Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, Fung-Leung WP. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004; 142:161–171. PMID: 15131002.
55. László V, Rothe G, Hegyesi H, Szeberényi JB, Orsó E, Schmitz G, Falus A. Increased histidine decarboxylase expression during in vitro monocyte maturation; a possible role of endogenously synthesised histamine in monocyte/macrophage differentiation. Inflamm Res. 2001; 50:428–434. PMID: 11556524.
56. Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005; 137:82–92. PMID: 15832054.
57. Wendeler M, Sandhoff K. Hexosaminidase assays. Glycoconj J. 2009; 26:945–952. PMID: 18473163.
58. Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012; 106:9–14. PMID: 22112783.
59. Pradalier A. Late-phase reaction in asthma: basic mechanisms. Int Arch Allergy Immunol. 1993; 101:322–325. PMID: 8324396.
60. Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent: partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991; 87:446–453. PMID: 1991831.
61. Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989; 142:927–931. PMID: 2464033.
62. Silvestri M, Bontempelli M, Giacomelli M, Malerba M, Rossi GA, Di Stefano A, Rossi A, Ricciardolo FL. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: markers of systemic inflammation? Clin Exp Allergy. 2006; 36:1373–1381. PMID: 17083347.
63. Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India. 2010; 27:66–71. PMID: 20616938.
64. Vo TH, Le NH, Patel MS, Phan LT, Tran Minh NN. Acute allergic reactions in Vietnamese children after drinking a new milk product. Foodborne Pathog Dis. 2012; 9:156–159. PMID: 22315953.
65. MacGlashan DW Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997; 158:1438–1445. PMID: 9013989.
66. Saini SS, MacGlashan DW Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FCεRIα surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999; 162:5624–5630. PMID: 10228046.
67. Nakano N, Nishiyama C, Tokura T, Nagasako-Akazome Y, Ohtake Y, Okumura K, Ogawa H. Procyanidin C1 from apple extracts inhibits Fc epsilon RI-mediated mast cell activation. Int Arch Allergy Immunol. 2008; 147:213–221. PMID: 18594151.
68. Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell FceR1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994; 269:29697–29703. PMID: 7961959.
69. Miura K, Lavens-Phillips S, MacGlashan DW Jr. Piceatannol is an effective inhibitor of IgE-mediated secretion from human basophils but is neither selective for this receptor nor acts on syk kinase at concentrations where mediator release inhibition occurs. Clin Exp Allergy. 2001; 31:1732–1739. PMID: 11696049.
70. Qin HD, Shi YQ, Liu ZH, Li ZG, Wang HS, Wang H, Liu ZP. Effect of chlorogenic acid on mast cell-dependent anaphylactic reaction. Int Immunopharmacol. 2010; 10:1135–1141. PMID: 20620227.
Fig. 1
Light microphotographs using inverted microscopy of rat peritoneal mast cells (RPMCs) in hydroxyethyl piperazineethanesulfonic acid-Tyrode buffer. RPMCs of normal group (A), RPMCs stimulated with compound 48/80 (B), RPMCs pretreated with disodium cromoglycate (100 µg/ml) (C), RPMCs pretreated with type-A procyanidine polyphenols from Cinnamomum zeylanicum (TAPP-CZ; 3 µg/ml) (D), RPMCs pretreated with TAPP-CZ (10 µg/ml) (E), and RPMCs pretreated with TAPP-CZ (30 µg/ml) (F). White arrows indicate mast cells.
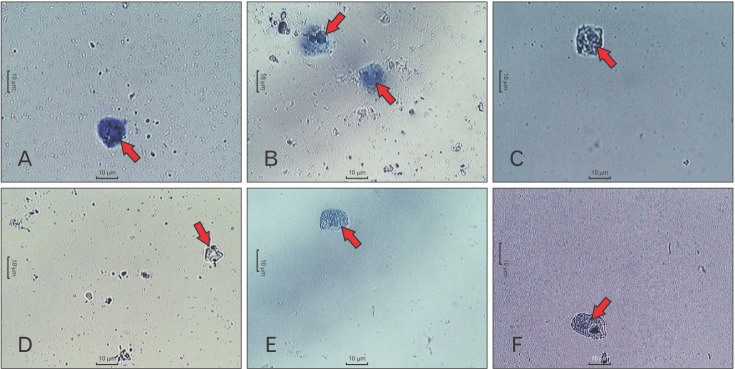
Fig. 2
Effect of type-A procyanidine polyphenols from Cinnamomum zeylanicum (TAPP-CZ) on compound 48/80 (C48/80)-induced histamine (A), β-hexosaminidase (β-HEX) (B), and interleukin 4 (IL-4) (C) release in rat peritoneal mast cells. Data was analyzed by one-way ANOVA followed by Dunnett's test. *P<0.05, **P<0.01, ***P<0.001 as compared to C48/80 control group and ###P<0.001 as compared to normal group. DSCG, disodium cromoglycate.
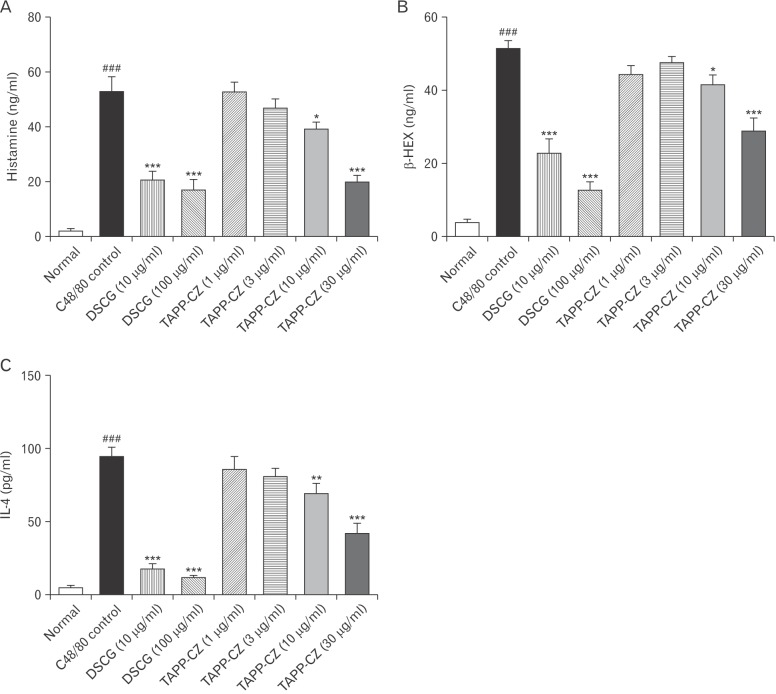
Table 1
Effect of TAPP-CZ on C48/80-induced mast cell degranulation
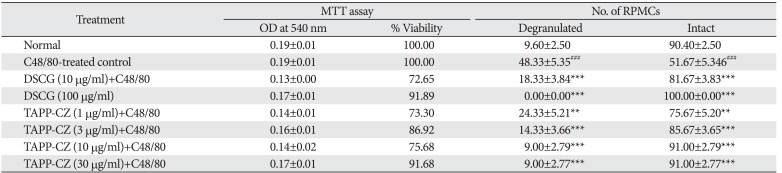
TAPP-CZ, type-A procyanidine polyphenols from Cinnamomum zeylanicum; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RPMC, rat peritoneal mast cell; DSCG, disodium cromoglycate; C48/80, compound 48/80. Data was analyzed by one-way ANOVA followed by Dunnett's test. **P<0.01, ***P<0.001 as compared to C48/80 control group and ###P<0.001 as compared to normal group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download