Abstract
Due to the importance of neural stem cells (NSCs) in plasticity of the nervous system and treating neurodegenerative diseases, the main goal of this study was to evaluate the effects of radiofrequency radiation emitted from a GSM 900-MHz mobile phone with different exposure duration on proliferation, differentiation and apoptosis of adult murine NSCs in vitro. We used neurosphere assay to evaluate NSCs proliferation, and immunofluorescence assay of neural cell markers to examine NSCs differentiation. We also employed alamarBlue and caspase 3 apoptosis assays to assess harmful effects of mobile phone on NSCs. Our results showed that the number and size of resulting neurospheres and also the percentage of cells differentiated into neurons decreased significantly with increasing exposure duration to GSM 900-MHz radiofrequency (RF)-electromagnetic field (EMF). In contrast, exposure to GSM 900-MHz RF-EMF at different durations did not influence cell viability and apoptosis of NSCs and also their astrocytic differentiation. It is concluded that accumulating dose of GSM 900-MHz RF-EMF might have devastating effects on NSCs proliferation and neurogenesis requiring more causations in terms of using mobile devices.
The explosively growing use of mobile phones has upraised worldwide substantial concerns about their potentially harmful effects on human health. The harmful effects of electromagnetic fields (EMF) emitted from sources such as mobile phones vary extensively depending on the frequency and intensity of the fields [1]. The frequency of EMF emitted by mobile phones ranges from 800 to 2000 MHz, categorized as the radiofrequency (RF) waves. According to the global system of mobile communication (GSM), 900 MHz is of the most widely used frequency band [2].
Placing the antennas near the head during cell phone usage and also the scattering waves generated by base stations cause propagation of radio waves into the central nervous system (CNS) [3]. Radio waves can initiate molecular responses which would result in altered cell proliferation or cell death [4]. In this regard, loss of neurons was detected in the hippocampus of 4-week-old rats as a consequence of repeated in utero exposure to GSM 900-MHz at a specific absorption rate (SAR) of 2 W/kg (1 hour daily, throughout prenatal life) [56]. Moreover, a single head-only exposure (15 minutes in duration) to GSM 900 MHz at a SAR of 6 W/kg was shown to be sufficient to decrease glutamate and gamma amino butyric acid receptors and trigger an astroglial reaction in adult rat brain [7].
Neural stem cells (NSCs) are present in the mammalian CNS as the life-long source of neurons and glia [8]. These cells are involved in active neurogenesis that occurs in two neurogenic zones namely the subventricular zone (SVZ) of the lateral ventricles and the sub-granular zone of the hippocampal dentate gyrus [910]. Interestingly, cells at different stage of development in the process of neurogenesis could be affected by various factors such as disease [11], drugs [11], diets [12], exercise [13], aging [14], and other physical stimuli such as EMFs [1516]. Therefore, due to the widespread use of mobile communication systems in daily life, determining the effect of RF-EMF radiation on proliferation, differentiation, and apoptosis of NSCs is vital. While some studies on cell lines and animal models demonstrated no effect of RF-EMF on proliferation, gene expression, and apoptosis [17], other studies reported the loss of neurons in the brain and also increase of apoptosis in embryonic NSCs after exposure to RF-EMF radiation [18]. According to the latest study to date, with the aim of assessing the effects of 1800-MHz radio wave on proliferation, differentiation, and apoptosis of embryonic NSCs, RF waves at different SAR values did not influence the rate of death, proliferation, and differentiation of these cells into neurons and astrocytes [19]. So far, no in-vitro research has been performed to evaluate the effects of the widely used GSM 900 MHz on the proliferation, differentiation, and apoptosis of NSCs. In addition, the works to date are mainly focused on the effects of varying SARs but not exposure time duration. Therefore, this study was aimed to evaluate the effects of RF radiation emitted from a GSM 900-MHz mobile phone on proliferation, differentiation, and apoptosis of SVZ derived NSCs in vitro at different time duration.
Adult male BALB/c mice (25–30 g) were utilized in this study (the Laboratory Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran).
Primary neurospheres were generated from the lateral wall of SVZ of the lateral ventricles using the neurosphere assay as described elsewhere [20]. Briefly, after anesthetizing the animals using 4% isoflurane and performing cervical dislocation, the brains were removed, washed several times with cold phosphate buffered saline (PBS) containing 10% penicillin/streptomycin. Then, the SVZ of both lateral ventricles were harvested from each brain under a dissection microscope. After chopping with a razor blade, the harvested tissue was digested in 0.05% trypsin-EDTA (37℃, 5–7 minutes) and then mechanically dissociated to achieve a single cell suspension. Cells harvested from each brain were re-suspended in a complete neurosphere medium (5 ml) supplemented with epidermal growth factor (EGF, 20 ng/ml), basic fibroblastic factor (bFGF, 10 ng/ml), and heparin (2 µg/ml). The suspension was plated in a T25 flask and incubated in a humidified incubator with 5% CO2 for 8 days. The resulting primary neurosphere were collected, centrifuged, and dissociated into single cells and replated in the complete neurosphere medium to expand NSCs for experiments.
To expose NSCs to GSM 900-MHz RF-EMF, a known number of single cells from passage 2 neurospheres were resuspended in complete neurosphere medium supplemented with growth factors in 15-ml conical tubes. The tubes were centrifuged gently for 30 seconds to precipitate the cells to the bottom of the tubes. The tubes were placed circularly in the far field distance around the GSM antenna to obtain uniform irradiation for 0 (control), 15 (group A), 30 (group B), 60 (group C), and 120 (group D) minutes. The samples were kept inside a tissue culture incubator with 37℃ temperature and 95% humidity during the irradiation period.
For all experiments, NSCs were irradiated with a GSM 900-MHz mobile simulator in a “Talk mode.” This mobile phone simulator was developed at Department of Medical Physics and Biomedical Engineering, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran by cooperation of the private sector [212223]. A detailed description of this system and its dosimetry is described in Table 1. The exposure set-up was controlled and monitored by a calibrated spectrum analyzer. During exposure, the temperature of the incubator was monitored and maintained at 37±0.1℃. Loose pellets of NSCs in the bottom of 15 ml tubes were placed circularly around the GSM antenna to obtain uniform irradiation. The samples were positioned in the far field. Far field was measured by using the following equation. Where d is the length of the GSM antenna and λ is the wave-length. The average SAR calculated for this experiment was 2.287 W/kg.
After reaching the end of each irradiation time points, the contents of each tube, representing each condition, was divided into eight wells of a 96-well plates at a density of 5,000 cells/200 µl of complete neurosphere medium supplemented with growth factors and incubated for 8 days to form neurospheres. Using an Olympus (Olympus, Center Valley, PA, USA) inverted microscope all neurospheres bigger than or approximately equal to 50 µm in diameter were counted [24] and recorded as the neurosphere forming frequency per group. At the same time, applying a systematic random sampling method, we captured 4–5 images from different locations of each well using an Olympus CKX41 digital camera to determine the mean diameter and surface area of neurospheres in each group using a stereological software (Stereo lite, SUMS, Shiraz, Iran). The mean for the number of neurospheres/group and mean neurosphere diameter (µm) were compared among all groups. All data were presented as mean±SEM for each group.
To evaluate the effects of GSM 900-MHz RF-EMF on differentiation capacity of NSCs, we plated irradiated cells from different groups in a differentiation assay known as the neuroblast assay [1025]. Briefly, cells (3×105 cells/ml) were plated in complete neurosphere medium supplemented with EGF (20 ng/ml), bFGF (10 ng/ml), and 5% fetal calf serum (FCS) in 24-well plates that were coated with poly-L-ornithine. The cultures were incubated for 3–4 days in a humidified incubator with 5% CO2 and then, their medium were replaced with the same medium containing 5% FCS but without growth factors. After 4 days, the cultures were fixed using cold paraformaldehyde (PFA; 4%, 20 minutes at room temperature) and processed for immunofluorescence analysis of neuronal and astrocytic markers.
Fixed samples were washed with PBS to remove PFA, and then the primary antibody solution containing mouse monoclonal anti-β-III-tubulin (1:1,000) and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; 1:1,000) in PBST (PBS+0.1% Triton-X) supplemented with 10% normal goat serum (NGS) was added to each well of the 24-well plates and incubated kept for 2 hours at room temperature. After removing the primary antibody and washing samples with PBS, the secondary antibody solution containing goat anti-mouse Alexa-Fluor 488 and goat anti-rabbit Alexa-Fluor 568 (1:500) in PBS-Triton supplemented with 10% NGS were added to each well and incubated for 45 minutes at room temperature in dark. DAPI (1:500) was also added to the secondary antibody solution to counterstain cell nuclei. Using a fluorescent microscope (Olympus IX-71) equipped with a Canon EOS digital camera (Canon, Tokyo, Japan) 10–15 representative pictures/well were taken and cells were counted after merging images using Adobe Photoshop CS4 and the data were presented as a percentage of total cells counted.
To assess the effect of GSM 900-MHz RF-EMF radiation on NSCs viability, cells from different treatment groups were plated in 96 well plates at the density of 5,000 cells/well in 200 µl of complete neurosphere medium supplemented with growth factors and incubated for 8 hours and then cell viability was measured using alamarBlue assay following the manufacturer's instructions. Briefly, 20 µl of the ready to use alamarBlue solution were added to each well and incubated for 2 hours at 37℃ till non-fluorescent resazurin was reduced to resorufin, a compound that is red in color and highly fluorescent. Then, the mean fluorescent intensity from each group was determined using Bio-Rad plate reader (Hercules, CA, USA) at 570 nm excitation wavelength.
To evaluate the possible deleterious effects of cell phone irradiation on initiation of cell death in NSCs, cells treated with 2.287 W/kg of GSM 900-MHz RF-EMF at 0, 15, 30, 60, and 120 minutes time points were collected, centrifuged and fixed with cold PFA (4%, 20 minutes at room temperature). After washing, cells were incubated in primary antibody solution (as described before) containing rabbit anti-activated caspase-3 (1:1,000) for 2 hours at room temperature. Then cells were centrifuged to remove antibody and washed three times with PBS. The samples were incubated with secondary goat anti-rabbit Alexa-Fluor 488 (1:500) for 1 hour at room temperature to label positive cells. After removing the antibody and washing with PBS, the samples were resuspended in PBS and analyzed with flow cytometry.
Long-term exposure to GSM 900 MHz decreases NSC proliferation. Results from the NSCs exposed to 2.287 W/kg RF-EMF for 0 (control), 15 (group A), 30 (group B), 60 (group C), and 120 (group D) minutes showed that while the mean neurosphere number/5,000 cells was 46.94±3.27 for the control group, this measure was 59.91±5.18 for group A, 49.06±4.11 for group B, 45.94±5.61 for group C, and 25.56±3.41 for group D. Statistical analysis revealed that exposure of the harvested NSCs to 2.287 W/kg RF-EMF led to a significant decrease in neurosphere formation in group D in comparison to the control and other treatment groups (Fig. 1A).
Additionally, the mean neurosphere diameter was 111.7±3.16 µm for the control group, and 113.5±2.84, 118 ±3.76, 106.3±3.07, and 96.8±2.88 µm for group A, B, C, and D, respectively. Statistical analysis revealed that exposure of the harvested NSCs to 2.287 W/kg RF-EMF would result in a significant reduction in the neurosphere diameter in group D comparing to the control, group A and B (Fig. 1B).
Overall, these data indicate that although neurospheres formed in all treatment groups (Fig. 1C), exposure to 2.287 W/kg RF-EMF for 120 minutes would significantly affect NSCs proliferative capacity.
Exposure to GSM 900 MHz decreases neuronal differentiation in NSCs. To analyze the effects of GSM 900-MHz RF-EMF exposure on NSC differentiation, the cells were first exposed to 2.287 W/kg RF-EMF at different exposure times as mentioned earlier and then plated in differentiation culture medium at a density of 3×105 cells/ml for 8 days. No significant difference was noticed between different treatment groups in the percent cells differentiated into GFAP+astrocytes (Fig. 2A, C). In contrast, by increasing RF-EMF exposure time a significant reduction in the percent cells differentiated into β-III-tubulin+ neurons was detected in all treatment groups (Fig. 2B, C). These data show that long-term exposure of NSCs might result in a dramatic decrease in their neuronal differentiation.
Exposure to GSM 900 MHz does not influence NSCs viability. After exposing NSCs to GSM 900-MHz RF-EMF the amount of cause cell death was measured using alamarBlue viability assay 8 hours after radiation. The results revealed no significant difference between different groups in terms of viability (Fig. 3A). In addition, we checked for expression of caspase 3 as an apoptosis marker 2 hours after radiation. Flow cytometric analysis for caspase 3 expression did not show any significant cell death post-irradiation to 2.287 W/kg GSM 900-MHz RF-EMF as compared to the control group (Fig. 3B, C).
Current study were planned to evaluate the effects of RF radiation emitted from a mobile phone GSM 900 MHz at SAR value of 2.287 W/kg on proliferation, differentiation and apoptosis of adult NSCs harvested from the SVZ of adult murine brain at different time durations. When sorting the groups in terms of time, we considered both short-term and long-term exposure to RF for an accurate assessment of the effects of GSM 900-MHz RF-EMF at various time intervals on the proliferation, differentiation, and apoptosis of neural stem and progenitor cells. Extensive number of studies on the effects of extremely low frequency (ELF)-EMF exposure on adult NSCs proliferation was performed at different frequencies. Most of these studies showed that ELF-EMF exposure would cause an increase in the proliferation and neural differentiation of NSCs [1516262728]. However, the effects of RF-EMF exposure on NSCs proliferation and differentiation are yet unknown from many aspects. We found that exposure to GSM 900-MHz RF-EMF at different durations did not influence cell viability and apoptosis of adult NSCs and also their astrocytic differentiation. Though, the number and size (i.e., diameter) of neurospheres and also the percentage of differentiated neurons have decreased significantly with increasing the durations of exposure to GSM 900-MHz RF-EMF. These results pave the way for better understanding of the potential effects of RF-EMF exposure on adult NSCs. The proliferation of NSCs is related to the self-renewal and genome replication ability, which is of great importance in maintaining the total number of NSCs for generating various types of neurons and glia during brain development. It has been shown that the size of neurospheres has a linear correlation with the number of cell divisions of the original neurosphere-forming cell and its progeny [29]. Accordingly, as the dose of RF increased, the size of the resulting neurospheres decreased. It seems that increasing the dose of RF also resulted in recruiting less NSCs into the pool of dividing cells as evidenced by less neurosphere formation in the high dose group. But, compared to the control group, the short term group (15 minutes) showed slight increase in the proliferation, i.e., the number of neurospheres. The increasing and decreasing trends in proliferation of NSCs seen in this study hypothesizes a biphasic behavior for RF. From the perspective of RF effect on proliferation of NSCs, it was lately reported that 1800-MHz RF-EMF exposure neither affected the embryonic NSC proliferation or the cell cycle, nor influenced the mRNA expression of the cell-cycle-related genes p21, p53, and GADD45. We presume that the different cell models, durations, and frequencies of exposure are the primary reasons for these conflicts. However, for a more reliable proposition, further studies are required.
The fate of neural and progenitor stem cells in the process of differentiation into neurons and glia is one of the most important procedure in the brain. To date, there is little evidence concerning the effects of RF-EMF exposure on neuro-gliogenesis in the brain. In this study, we found that a higher dose or a much longer period of GSM 900-MHz RF-EMF exposure on undifferentiated NSCs decreased the ratio of differentiated neurons but had no effect on the ratio of NSCs differentiated into astrocytes. Many studies indicate that neuron may be the main target of RF-EMF, hence affecting neurogenesis in the brain. For example neuron loss induced in adult rats when exposed to 900-MHz EMF, has also been reported in several studies [3183031]. Studying the effects of 1800-MHz radio waves on embryonic NSCs showed that 1800-MHz RF-EMF radiation would cause damage and impairment in the expression of helix-loop-helix genes, essential for neuronal development [19]. All these results certainly indicate that EMF-RF may affect neurogenesis of NSCs. However, the precise fundamental cellular and molecular mechanisms are unknown and further in vivo and in vitro studies are still required.
Apoptosis occurs in response to a wide variety of environmental stimuli [323334]. We found that RF-EMF GSM 900-MHz exposure at a SAR of 2.287 W/kg has no effect on the cell viability and apoptosis of NSCs, verified by alamarBlue viability test and anti-caspase-3 immunofluorescence, respectively. The results of these two tests confirmed each other. Our results regarding both cell viability and apoptosis, are in line with many previous studies on cell lines and animal models, demonstrated no effects of RF-EMF on apoptosis of neural cells [17]. As reported in a late study, a SAR of 4 W/kg 1800-MHz RF-EMF exposure has no effect on the cell viability and apoptosis of embryonic NSCs using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and Hoechst 33342 staining. No change in the percentage of TUNEL positive cells was found. In addition, the results from the Hoechst 33342 staining showed no change in cell death after RF-EMF exposure. No change in the mRNA expression of apoptosis-related genes Bax and Bcl-2 after RF-EMF exposure was inspected [35]. In contrast, a previous study has reported that 1950-MHz RF-EMF exposure may cause transcriptional changes in apoptosis related genes, such as Bax [36]. Also the up-regulation of apoptosis-related genes have also been previously observed in embryonic stem cell derived neural progenitor cells in vitro [19]. These conflicts may be due to different cell models, exposure SARs, durations, and frequencies of exposure that needs further investigation. Overall, it seems accumulating dose of GSM 900-MHz RF-EMF might induce devastating effects on NSCs proliferation and neurogenesis which implicates a more cautious use of mobile phones especially in long durations.
Figures and Tables
Fig. 1
Effect of GSM 900 MHz on proliferation of adult neural stem cells in vitro. (A) Mean neurosphere forming frequency. (B) Mean neurosphere diameter. (C) Representative neurospheres formed in different treatment groups. Scale bar=100 µm. **P<.01, ***P<0.001.
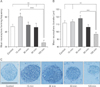
Fig. 2
Effect of GSM 900 MHz on differentiation of neural stem cell. (A) Mean percentage of astrocytes. (B) Mean percentage of neurons. (C) Representative immunostained pictures from the control and 120-minute treatment groups. GFAP, glial fibrillary acidic protein; IR, immunoreactive. Scale bar=100 µm. *P<0.05, ***P<0.001.
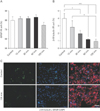
Fig. 3
Effect of GSM 900 MHz on cell viability and apoptosis. (A) Cell viability of neural stem cells using alamarBlue assay. (B) Mean percentage of caspase 3 apoptotic cells in different treatment groups. (C) Representative flow cytometry plot analyzing caspase 3 immunoreactive cells.

Table 1
The exposure system and physical properties of GSM 900-MHz mobile simulator

Acknowledgements
This study was carried out by Mrs. Mahsa Eghlidospour to obtain master degree in Medical Physics and was financed by the Shiraz University of Medical Sciences (grant 7136-93). This project was jointly supported by the Ionizing and Non-ionizing Radiation Protection Research Center (INIRPRC) and Neural Stem Cell & Regenerative Neuroscience laboratory at Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. The authors would like to thank Dr. Tahereh Esmaeilpour for her logistical support, Dr. Ali Zamani for his advice and critics on research design, and Dr. Ali Soleimani for his contribution in data analysis.
References
1. Feychting M, Ahlbom A, Kheifets L. EMF and health. Annu Rev Public Health. 2005; 26:165–189.
2. Wu S, Razavi B. A 900-MHz/1.8-GHz CMOS receiver for dual-band applications. IEEE J Solid-State Circuits. 1998; 33:2178–2185.
3. Bas O, Odaci E, Kaplan S, Acer N, Ucok K, Colakoglu S. 900 MHz electromagnetic field exposure affects qualitative and quantitative features of hippocampal pyramidal cells in the adult female rat. Brain Res. 2009; 1265:178–185.
4. Moulder JE, Erdreich LS, Malyapa RS, Merritt J, Pickard WF, Vijayalaxmi . Cell phones and cancer: what is the evidence for a connection? Radiat Res. 1999; 151:513–531.
5. Odaci E, Bas O, Kaplan S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain Res. 2008; 1238:224–229.
6. Bas O, Odaci E, Mollaoglu H, Ucok K, Kaplan S. Chronic prenatal exposure to the 900 megahertz electromagnetic field induces pyramidal cell loss in the hippocampus of newborn rats. Toxicol Ind Health. 2009; 25:377–384.
7. Mausset-Bonnefont AL, Hirbec H, Bonnefont X, Privat A, Vignon J, de Sèze R. Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiol Dis. 2004; 17:445–454.
8. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992; 255:1707–1710.
9. Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005; 85:523–569.
10. Azari H, Reynolds BA. In vitro models for neurogenesis. Cold Spring Harb Perspect Biol. 2016; 8:a021279.
11. Lee TH, Lee CH, Kim IH, Yan BC, Park JH, Kwon SH, Park OK, Ahn JH, Cho JH, Won MH, Kim SK. Effects of ADHD therapeutic agents, methylphenidate and atomoxetine, on hippocampal neurogenesis in the adolescent mouse dentate gyrus. Neurosci Lett. 2012; 524:84–88.
12. Utsugi C, Miyazono S, Osada K, Sasajima H, Noguchi T, Matsuda M, Kashiwayanagi M. Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding. PLoS One. 2014; 9:e97309.
13. Yagita Y, Kitagawa K, Sasaki T, Terasaki Y, Todo K, Omura-Matsuoka E, Matsumoto M, Hori M. Postischemic exercise decreases neurogenesis in the adult rat dentate gyrus. Neurosci Lett. 2006; 409:24–29.
14. Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, Berninger B. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron. 2015; 85:710–717.
15. Abbasnia K, Ghanbari A, Abedian M, Ghanbari A, Sharififar S, Azari H. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 2015; 48:104–113.
16. Sherafat MA, Heibatollahi M, Mongabadi S, Moradi F, Javan M, Ahmadiani A. Electromagnetic field stimulation potentiates endogenous myelin repair by recruiting subventricular neural stem cells in an experimental model of white matter demyelination. J Mol Neurosci. 2012; 48:144–153.
17. Joubert V, Leveque P, Cueille M, Bourthoumieu S, Yardin C. No apoptosis is induced in rat cortical neurons exposed to GSM phone fields. Bioelectromagnetics. 2007; 28:115–121.
18. Sonmez OF, Odaci E, Bas O, Kaplan S. Purkinje cell number decreases in the adult female rat cerebellum following exposure to 900 MHz electromagnetic field. Brain Res. 2010; 1356:95–101.
19. Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, Schuderer J, Kuster N, Wobus AM. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005; 19:1686–1688.
20. Azari H, Rahman M, Sharififar S, Reynolds BA. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J Vis Exp. 2010; (45):pii: 2393.
21. Mortazavi S, Mosleh-Shirazi M, Tavassoli A, Taheri M, Mehdizadeh A, Namazi S, Jamali A, Ghalandari R, Bonyadi S, Haghani M, Shafie M. Increased radioresistance to lethal doses of gamma rays in mice and rats after exposure to microwave radiation emitted by a GSM mobile phone simulator. Dose Response. 2013; 11:281–292.
22. Mortazavi SM, Erfani N, Mozdarani H, Azmoonfar R, Shokrpour N. Induction of apoptosis by 900 MHz radiofrequency radiation emitted from a GSM mobile phone simulator in bystander Jurkat cells. Int J Radiat Res. 2015; 13:181–186.
23. Kazemi E, Mortazavi SM, Ali-Ghanbari A, Sharifzadeh S, Ranjbaran R, Mostafavi-Pour Z, Zal F, Haghani M. Effect of 900 MHz electromagnetic radiation on the induction of ROS in human peripheral blood mononuclear Cells. J Biomed Phys Eng. 2015; 5:105–114.
24. Hamedi A, Ghanbari A, Razavipour R, Saeidi V, Zarshenas MM, Sohrabpour M, Azari H. Alyssum homolocarpum seeds: phytochemical analysis and effects of the seed oil on neural stem cell proliferation and differentiation. J Nat Med. 2015; 69:387–396.
25. Azari H, Sharififar S, Darioosh RP, Fortin JM, Rahman M, Reynolds BA. Purifying immature neurons from differentiating neural stem cell progeny using a simple shaking method. J Stem Cell Res Ther. 2014; 4:178.
26. Cuccurazzu B, Leone L, Podda MV, Piacentini R, Riccardi E, Ripoli C, Azzena GB, Grassi C. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp Neurol. 2010; 226:173–182.
27. Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008; 215:129–139.
28. Leone L, Fusco S, Mastrodonato A, Piacentini R, Barbati SA, Zaffina S, Pani G, Podda MV, Grassi C. Epigenetic modulation of adult hippocampal neurogenesis by extremely low-frequency electromagnetic fields. Mol Neurobiol. 2014; 49:1472–1486.
29. Mori H, Ninomiya K, Kino-oka M, Shofuda T, Islam MO, Yamasaki M, Okano H, Taya M, Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res. 2006; 84:1682–1691.
30. Salford LG, Brun AE, Eberhardt JL, Malmgren L, Persson BR. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ Health Perspect. 2003; 111:881–883.
31. Masuda H, Ushiyama A, Takahashi M, Wang J, Fujiwara O, Hikage T, Nojima T, Fujita K, Kudo M, Ohkubo C. Effects of 915 MHz electromagnetic-field radiation in TEM cell on the blood-brain barrier and neurons in the rat brain. Radiat Res. 2009; 172:66–73.
32. Wickman G, Julian L, Olson MF. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012; 19:735–742.
33. Abdanipour A, Tiraihi T, Noori-Zadeh A, Majdi A, Gosaili R. Evaluation of lovastatin effects on expression of anti-apoptotic Nrf2 and PGC-1alpha genes in neural stem cells treated with hydrogen peroxide. Mol Neurobiol. 2014; 49:1364–1372.
34. Zhang S, Chen X, Yang Y, Zhou X, Liu J, Ding F. Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Mol Cell Biochem. 2011; 354:161–170.
35. Chen C, Ma Q, Liu C, Deng P, Zhu G, Zhang L, He M, Lu Y, Duan W, Pei L, Li M, Yu Z, Zhou Z. Exposure to 1800 MHz radiofrequency radiation impairs neurite outgrowth of embryonic neural stem cells. Sci Rep. 2014; 4:5103.
36. Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH, Liu HS, Zhu H, Cheng JD, Liu YL, Li AM, Zhang Y. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS One. 2012; 7:e42332.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download