Abstract
In assisted reproductive techniques, the operator attempts to select morphologically best embryos to predict embryo viability. Development of polarized light microscope, which evaluates the oocytes' spindles according to birefringence of living cells, had been helpful in oocyte selection. The aim of this study is evaluating the relationship between meiotic spindles visualization and intracytoplasmic sperm injection (ICSI) outcomes in human oocytes. In this study, 264 oocytes from 24 patients with an average age of 30.5±7.5 years with infertility duration of 1 to 10 years were collected. The oocytes were randomly allocated to the control injection group (n=126) and the oocyte imaging group (spindle-aligned group) (n=138). In the spindle-aligned group, the meiotic spindle was identified by means of polarized light microscope to align the spindle at 6 or 12 o'clock. Then the spindle-aligned group was divided into three sub-groups based on spindle morphology: fine, average, and (poor). After ICSI, embryos were checked every 24 hours and scored; 72 hours later, high-grade embryos were transferred intravaginally to uterus. This study showed that the fertilization rate in the spindle-aligned group was higher than the control group (P<0.05). After cleavage, a positive correlation was observed between spindle morphology and embryo morphology. Among the sub-groups of spindle-aligned group, the embryos' morphology of the fine group was better than the other subgroups and embryos of the poor group had lower quality and more fragmentation. The results revealed that the selection of oocytes based on meiotic spindle imaging can significantly improve the rate of fertilization and embryo cleavage and certainly increase the rate of implantation.
Since the first healthy child was conceived via intracytoplasmic sperm injection (ICSI) [1], this technique has become increasingly popular as producing viable embryos having high implantation potentials. In addition, in in-vitro fertilization, the operator attempts to select the morphologically best embryo to predict embryo viability as to guide selection of embryos for implantation.
In the Assisted Reproductive Techniques (ART) Laboratory, embryo formation influenced by extracellular morphological characteristics of oocyte, such as zona pellucida, polar body, cumulus oophorus, and intracellular properties have been related to fertilization, cleavage, embryo-formation, and clinical outcomes [2345].
Oocyte chromosomes at metaphase II (MII) stage are aligned at the equatorial region of the meiotic spindle (MS). This structure plays a vital role in the sequence of events leading up to the completion of meiosis and fertilization and thus is a key determinant of oocyte developmental potential. The MS microtubules, which are responsible for proper separation of chromosomes, are highly sensitive to physical and chemical changes that may occur during oocyte retrieval and handling. It has been shown that the oocyte exposure to slight temperature fluctuations dramatically affects microtubular structure, with deleterious consequences on chromosomal organization [678]. Other parameters, such as increasing maternal age [910] and oocyte in in vitro aging is associated with abnormalities in MS architecture [8]. The most potentially dramatic consequences of MS changes are unbalanced separation and/or non-separation of chromatids, chromosome scattering, and the formation of aneuploidy embryos [91112].
The MS, through the different stages of meiosis, controls chromosome movement, and is involved in various functions which are essential for fertilization and early postfertilization events. These include the accountability for proper chromosome segregation and genomic stability after oocyte activation [1314].
The zona pellucida is a unique extracellular coat that cinctures the maturing oocyte during ovulation, fertilization, and first embryo development [15]. A correlation between zona birefringence and the potential for an embryo to develop to the blastocyst stage has already been shown [1516].
Using the routine methods for observing the spindle, such as immunohistochemistry and electron microscopy are limited to fixed specimens. The key to improve the techniques of prediction of oocyte developmental potential may lie in advances in quantitative imaging of the MS. Development of a polarized light microscope which evaluates the birefringence of living cells enables the evaluation of oocyte spindles without damaging the cell, as spindles are highly birefringent [517]. In addition to the MS, polarized light microscopy enables the evaluation of other sub-cellular oocyte structures, such as zona pellucida birefringence [18]. In this study, we aim to evaluate the relationship between MS visualization, morphology and ICSI outcomes in human oocytes.
Twenty-four couples who were undergoing ICSI cycles at highly specialized Jihad Daneshgahi Infertility Treatment Center, Qom branch (ACECR Center for Infertility Treatment, Qom Branch) entered the study. Couples that were selected for this study met the following criteria:
Age under 38-years old, retrieval of at least 5 and at most 15 mature oocytes, absence of male factor and injection of oocytes 36–40 hours following human chorionic gonadotropin (hCG) administration.
The trial design was approved by the Ethics Committee (registry No. 03-312) and all couples were required to sign a written consent before initiation of the treatment cycles.
Written informed consent was obtained from patients in which they agreed to share the outcomes of their own cycles for research purposes.
Patients divided into two groups: control microinjection group (polar body–aligned or PB-aligned) and treatment microinjection group (spindle-aligned). The control group has the oocyte aligned by the first polar body, a cell that separates from an oocyte during meiosis and contains a nucleus produced in meiotic division and very little cytoplasm, at the 6 or 12 o'clock position and the true location of the MS is unknown and sperm was injected at 3 o'clock. The MS of the spindle-aligned group was detected by polarized light microscope (CRI, Woburn, MA, USA) to align the spindle at 6 or 12 o'clock. Fertilization rate and embryo quality were compared between the oocyte groups. Our criteria was retrieving five mature oocytes, so patients with less than five mature oocytes were rulled out, since it was considered that there may not be a sensible chance of getting at least one embryo developing in each treatment group [19]. Surgically-retrieved-sperm patients undergoing ICSI was another factor of rulling out the patients.
Protocol of ovulation induction in all of the patients was standard long protocol. Pituitary desensitization with a gonadotropin-releasing hormone agonist and ovarian stimulation with gonadotropins (Merional, IBSA, Lugano, Switzerland), were carried out. The patient monitoring was done by transvaginal ultrasound and hCG was injected when at least three follicles more than 18 mm in diameter were seen in ultrasonography. Oocytes were recovered transvaginally under ultrasound guidance and ICSI was performed.
Ejaculated spermatozoa were obtained by masturbation after 3–5 days of ejaculatory abstinence. After liquefaction of semen at room temperature, sperm samples were prepared by discontinuous density-gradient centrifugation. For discontinuous density-gradients, the bottom fraction was aspirated and washed with Quinn's sperm wash medium (ART-1006, SAGE BioPharma, Trumball, CT, USA) twice at 2,500 rpm for 4 minutes and incubated at 37℃.
After retrieval, collected oocytes were incubated in culture medium (SAGE BioPharma) which was covered with mineral oil (Reproline, Rheinbach, Germany), at 37℃ and 6% CO2 for 3 hours. Cumulus cells were removed by 30-second exposure to 30 IU/ml hyaluronidase (SAGE BioPharma) in HEPES buffered human tubal fluid (HTF) followed by washing with HEPES-buffered HTF containing 5 mg/ml human serum albumin (SAGE BioPharma). The coronal cells were carefully removed by use of a series of finely drawn glass Pasteur pipettes [19]. Afterwards denuded oocytes were assessed for their structural integrity and meiotic maturity. The oocytes were randomized by using a random numbers table into the control and a treatment group and placed into fertilization medium (SAGE BioPharma) which was covered with mineral oil (Reproline), to await microinjection at 37℃ and 6% CO2.
PB-aligned oocytes were transferred into 4 µl of warm HEPES buffered HTF containing 5 mg/ml human serum albumin (SAGE BioPharma), during microinjection and image analysis (Reproline). Sperm were placed in a 4 µl polyvinylpyrrolidone solution (LifeGlobal, Guilford, CT, USA) in the center of microinjection dish immediately before sperm injection; oocytes were placed under an inverted microscope (IX71, Olympus, Tokyo, Japan) with a heated stage at 37.0±0.5℃ and observed at ×400 magnification. Then the sperm was injected to the mature oocyte which its PB was in 12 or 6 o'clock position and then transferred to cleavage medium (SAGE BioPharma) and placed at 37℃ and 6% CO2. The PolScope optical (CRi'sPolScope Technology) requirements for the spindle-aligned oocytes (treatment group) necessitated a sterile, disposable, glass-bottomed dish (FluoroDish, Sarasota, FL, USA) to be used during spindle location and microinjection. The oocytes were classified as normal and abnormal spindle morphologically. Following microinjection and imaging, both oocyte groups were transferred to equilibrated individual 50 µl droplets for culture in cleavage media (SAGE BioPharma) in a sterile plastic dish in an incubator supplied with 6% CO2 at 37℃.
Temperature during oocyte and embryo manipulation was strictly maintained for all oocytes and embryos at 37℃.
Fertilization was assessed 16–20 hours after microinjection. All oocytes with an environmental chamber to maintain the temperature during imaging were returned to an inverted microscope [19].
Normally fertilized oocytes were returned to 50 µl individual cultural droplets of equilibrated cleavage medium (SAGE BioPharma). In order to check the presence of a 2-cell cleavage plane, embryos from the spindle aligned group were assessed 26–28-hour postmicroinjection [19]. If division had occurred, embryos were transferred to 4 ml HEPES droplets and aligned and re-imaged as above. Embryo morphology grading was performed 70±2 hours after microinjection when all embryos (control and treatment) were re-imaged. Embryo transfer was performed trans-cervically 2±3 hours later, utilizing a double catheter (COOK, Bloomington, IN, USA).
Alignment protocols images and definitions of early embryo development determining the morphology of embryos were obtained after microinjection on day 3 before transferring the embryo. This time allows evaluation of cleavage speed and embryo morphology and is considered a standard time for all embryos [19].
Following detecting the fragmentation percentage, morphology of the embryos were graded as: grade 1, spherical embryos with equal-sized blastomeres and no extra cellular fragmentation; grade 2, embryos with <10% fragmentation; grade 3, embryos with 30% fragmentation; and grade 4, embryos with >50% fragmentation. The number of zygotes showing pronucleus divided by the number of oocytes that survived the injection process was expressed as the fertilization rate for both groups, as oocytes can be abolished by the microinjection process and it's independent of the spindle presence [19].
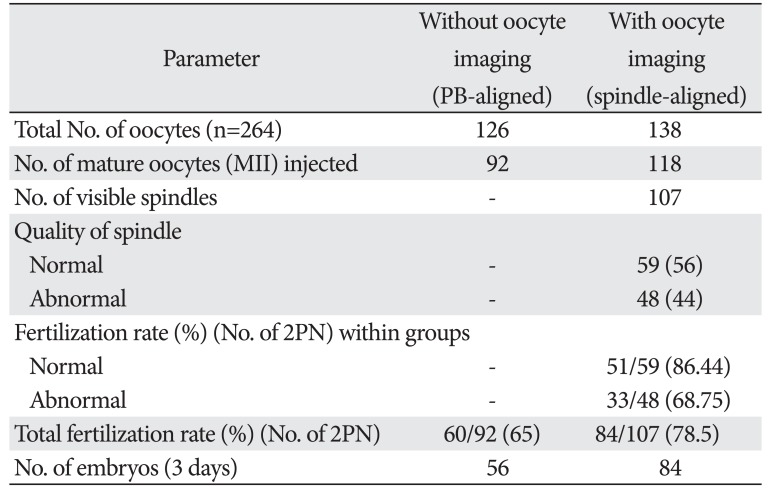
The oocytes of 24 patients were collected in this trial, with an average age of 30.5±7.5 years (range, 23–38 years), and a duration of infertility between 1 to 10 years. All the 264 oocytes were randomly allocated to the control injection group (n=126) and to the spindle injection group (n=138) (Table 1).
Out of the 138 oocytes allocated to the spindle-aligned group, 118 oocytes were MII (85%), 10 oocytes had no visible MS (7%), two oocyte had double spindle (2%) (Fig. 1C), and nine oocytes were in telophase stage (6%). There were 107 leaving oocytes (90%) with different visible spindle morphology at the time of microinjection. Different morphologies of visible spindle classified to normal 59/107 (55%) and abnormal 48/107 (45%) groups. Among these spindle-identified oocytes, 62.8% (67/107) of the first PBs were above the spindle or the lower region PB, and 6.9% (8/107) of the first PBs were in the opposite hemisphere to the spindle which is inserted into a poor group (Fig. 1).
After 24 hours, embryos pronuclei (PN) were checked. The percentage of embryos with appropriate PN morphology was significantly higher among embryos derived from oocytes in which MS was viewed (78%) than among embryos derived from oocytes in which the MS was not checked (65%). In addition, fertilization rates were higher in normal MS morphology than others (Fig. 2). It should be noted that after ICSI in spindle-aligned group, the number of dead oocytes was reduced.
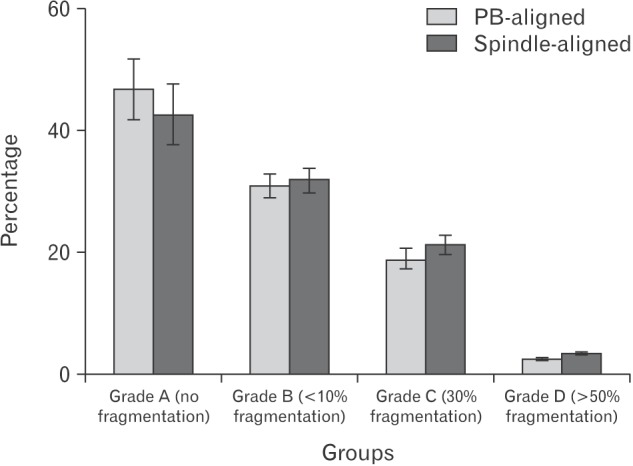
After 72 hours, morphology of embryos was checked and scored according to their quality. Embryos were divided into four groups 1–4. In spindle-aligned group, 47% (40/84) had grade 1, 31% (26/84) had grade 2, 19% (16/84) had grade 3, and 2.3% (2/84) had grade 4. In PB-aligned group, 42.8% (24/56) had grade A, 32% (18/56) had grade B, 21.4 (12/56) had grade C, and 3.5% (2/56) had grade D.
The identification of predictive markers for oocyte developmental potential prior to fertilization is one of the most studied areas in assisted reproductive techniques. Up to now, a few predictive non-invasive markers for oocyte quality have been known on the basis of morphological criteria, which can be evaluated using conventional microscopy [20]. Our study indicated that the use of the polarization light microscopy is non-invasive, leading to reduced oocyte death after ICSI. Non-invasive imaging of the MS, enhances spindle quality, increases fertility and improves the quality of the embryos in comparison to the control group. Oldenbourg [21] mentioned that the introduction of polarization light microscopy enabled the non-invasive visualization of sub-cellular structures in oocytes such as the MS and zona pellucida birefringence.
Tomari et al. [22] and Keefe et al. [23] showed that polarization light microscopy for non-invasive imaging of the MS has become an important item in the laboratory to assess the competence of gametes.
Previous methods of imaging the MS, such as immunohistochemistry and electron microscopy were limited to clinical use. Since the polarization light microscopy is less sensitive than optical microscopes, it has been underused in embryology. Furthermore polarizing microscopes with higher safety for women undergoing ICSI treatment, help the thermodynamic stability of the egg during ICSI [23].
Polarized light microscope imaging helps to detect more oocytes and mature oocytes in MII in comparison to the control group. Also, this type of imaging helps to observe the spindle better. Assessment of MS normality is of a strong predictive value for pregnancy chances of a particular oocyte. Its true value in the selection of the “best” embryo for embryonic transfer needs to be confirmed by a randomized controlled study.
Those oocytes are more likely to result in an ongoing pregnancy. Evidence is mounting that the formation of the MS at 39–40.5 hours after exposure to increasing levels of luteinizing hormone (or its surrogate hCG) in MII oocytes is a sign of oocyte competency [24]. Presence of an MS has been associated with higher fertilization and pregnancy rates [1625]. In the MII stage oocyte, chromosomes are aligned in the center of the MS, which is involved in many functions that are essential for the sequence of events leading to meiosis completion and fertilization [14]. However, recent observations with more sensitive imaging capacity of the latest polarized light microscopy systems have shown spindles morphology and the uniformity of their birefringence. Therefore, categorization of the MS into normal spindle and abnormal spindle has become feasible. Abnormal spindles (Fig. 1F) have been associated with reduced fertilization rates. Several studies [1726] indicate the importance of presence of a detectable MS in the oocyte cytoplasm prior to ICSI. In this study, a clear positive correlation between MS visualization, fertilization rate, and/or embryo development was described. It should be noted that this method reduces the number of dead oocytes after ICSI. The absence of a recognizable MS and the consequent oocyte (Fig. 1E) developmental impairment may be primarily attributed to oocyte immaturity [812]. It has been hypothesized that the lack of MS formation can be the result of aberrant signaling pathways or low energy supply during oocyte growth, resulting in both nuclear and cytoplasmic immaturity [1227]. Moreover, some oocytes were found to be clearly immature at the stage of telophase I when observed with the spindle view system [81028]. At this stage there is continuity between the ooplasm and the cytoplasm of the forming first PB (Fig. 1G). Therefore, the spindle view system allows inaccurate determination of oocyte's nuclear maturity and selection of fully mature eggs is directly involved in the subsequent fertilization process. It is well documented that physical and chemical changes in the condition of the spindle can lead to failed or disrupted fertilization and subsequent development [7].
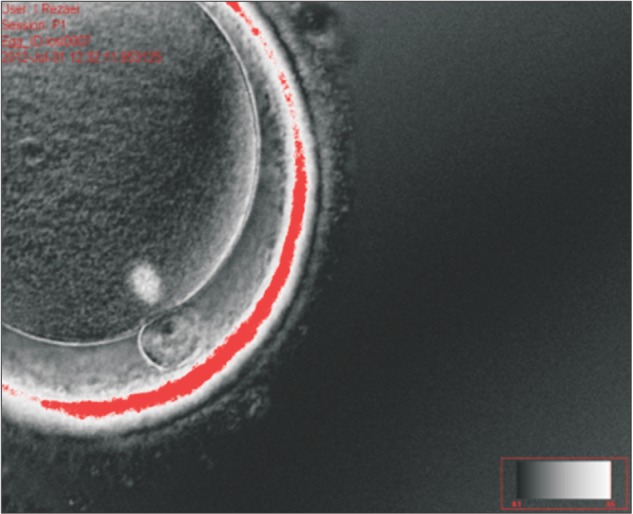
Long-term work with polarized light microscopy has resulted in the development of a definition of a “normal” spindle as having a barrel shape with clearly delineated boundaries and even distribution of birefringence (Fig. 4). These criteria can readily be used in the clinical setting with appropriate training of the embryologists.
De Sutter et al. [29] noted that the relationship between the morphology of oocytes and fertility success rate of ICSI program does not exist. Although some studies have demonstrated that morphologically normal oocytes are the most important factor in the production of high-grade embryos [30]. Furthermore, the results of Khalili et al. [31] showed that an oocyte with normal morphology not only significantly improves fertility rates, but also, with better production, ameliorates embryos in ART programs. This study indicates that imaging the MS morphology with a polarizing microscope can lead to better ICSI output in human oocytes. Furthermore, our results showed that spindle morphology and position of MS could predict fertilization rate and embryo morphology. In other studies, it is reported that close position of the MS to the PB was correlated with fertilization and cleavage rates and early embryo development and quality [19]. According to Rienzi et al. [28] high degrees of misalignment between the MS and the first PB increased risk of fertilization abnormalities. However, when normal fertilization occurred in such oocytes, the cleavage ability of developing embryos was not disturbed [28] and so Shen et al. [32] found that better pronuclear scores and higher pregnancy rates correlated with higher retardance [33]. However, in some researches, there was no significant correlation between the spindle retardance and embryo quality [34].
Polarized light microscopy, increases fertility and improves the quality of the embryos. It can improve the performance of laboratory and ICSI studies. Since fluorescent microscope can provide detailed information about chromosome and MS, further studies comparing polarizing and fluorescent microscope imaging methods seem necessary.
Acknowledgements
We would like to thank ACECR center for infertility treatment (Qom branch) for supporting this research project.
References
1. Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992; 340:17–18. PMID: 1351601.
2. Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006; 12:608–615. PMID: 16790106.
3. Borini A, Lagalla C, Cattoli M, Sereni E, Sciajno R, Flamigni C, Coticchio G. Predictive factors for embryo implantation potential. Reprod Biomed Online. 2005; 10:653–668. PMID: 15949227.
4. Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann N Y Acad Sci. 2004; 1034:132–144. PMID: 15731306.
5. Petersen CG, Oliveira JB, Mauri AL, Massaro FC, Baruffi RL, Pontes A, Franco JG Jr. Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod Biomed Online. 2009; 18:235–243. PMID: 19192344.
6. Sathananthan AH, Trounson A, Freemann L, Brady T. The effects of cooling human oocytes. Hum Reprod. 1988; 3:968–977. PMID: 3204152.
7. Wang WH, Sun QY. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front Biosci. 2006; 11:620–636. PMID: 16146756.
8. Gardner DK, Weissman A, Howles CM, Shoham Z. Clinical perspectives. Textbook of assisted reproductive techniques. 4th ed. Vol. 2. Boca Raton, FL: CRC Press;2012.
9. Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996; 11:2217–2222. PMID: 8943533.
10. Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001; 16:1464–1468. PMID: 11425830.
11. Bernard A, Fuller BJ. Cryopreservation of human oocytes: a review of current problems and perspectives. Hum Reprod Update. 1996; 2:193–207. PMID: 9079413.
12. Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: possible damage to the spindle apparatus. Reprod Biomed Online. 2002; 5:117–124. PMID: 12419035.
13. Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, Lessing JB, Amit A, Azem F. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004; 19:649–654. PMID: 14998965.
14. Rienzi L, Ubaldi F, Iacobelli M, Romano S, Minasi MG, Ferrero S, Sapienza F, Baroni E, Greco E. Significance of morphological attributes of the early embryo. Reprod Biomed Online. 2005; 10:669–681. PMID: 15949228.
15. Familiari G, Heyn R, Relucenti M, Sathananthan H. Structural changes of the zona pellucida during fertilization and embryo development. Front Biosci. 2008; 13:6730–6751. PMID: 18508691.
16. Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online. 2007; 14:166–174. PMID: 17298718.
17. Madaschi C, Aoki T, de Almeida Ferreira Braga DP, de Cássia Sávio Figueira R, Semião Francisco L, Iaconelli A Jr, Borges E Jr. Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod Biomed Online. 2009; 18:681–686. PMID: 19549448.
18. Pelletier C, Keefe DL, Trimarchi JR. Noninvasive polarized light microscopy quantitatively distinguishes the multilaminar structure of the zona pellucida of living human eggs and embryos. Fertil Steril. 2004; 81(Suppl 1):850–856. PMID: 15019819.
19. Cooke S, Tyler JP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod. 2003; 18:2397–2405. PMID: 14585893.
20. Omidi M, Khalili MA, Nahangi H, Ashourzadeh S, Rahimipour M. Does women's age influence zona pellucida birefringence of metaphase IotaIota oocytes in in-vitro maturation program? Iran J Reprod Med. 2013; 11:823–828. PMID: 24639703.
21. Oldenbourg R. A new view on polarization microscopy. Nature. 1996; 381:811–812. PMID: 8657288.
22. Tomari H, Honjou K, Nagata Y, Horiuchi T. Relationship between meiotic spindle characteristics in human oocytes and the timing of the first zygotic cleavage after intracytoplasmic sperm injection. J Assist Reprod Genet. 2011; 28:1099–1104. PMID: 21882015.
23. Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online. 2003; 7:24–29. PMID: 12930570.
24. Kilani S, Cooke S, Chapman M. The change of the meiotic spindle over time: a possible marker to optimise ICSI? Aust N Z J Obstet Gynaecol. 2006; 46(S2):A14–A15.
25. Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod Biomed Online. 2009; 18:674–680. PMID: 19549447.
26. Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reprod. 2001; 16:2374–2378. PMID: 11679523.
27. Fauser BC. Step-down follicle-stimulating hormone regimens in polycystic ovary syndrome. In : Filicori M, Flamigni C, editors. Ovulation Induction: Basic Science and Clinical Advances. Amsterdam: Elsevier;1994. p. 153–162.
28. Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, Tesarik J, Greco E. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003; 18:1289–1293. PMID: 12773461.
29. De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996; 11:595–597. PMID: 8671274.
30. Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997; 12:1750–1755. PMID: 9308806.
31. Khalili MA, Mojibian M, Sultan AM. Role of oocyte morphology on fertilization and embryo formation in assisted reproductive techniques. Middle East Fertil Soc J. 2005; 10:72–77.
32. Shen Y, Stalf T, Mehnert C, De Santis L, Cino I, Tinneberg HR, Eichenlaub-Ritter U. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod Biomed Online. 2006; 12:737–751. PMID: 16792851.
33. Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998; 13:154–160. PMID: 9512249.
34. De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005; 11:36–42. PMID: 16102284.
Fig. 1
(A) The oocyte spindle is located between 0° and 60° relative to the polar body. (B) The oocyte spindle is located between 60° and 120° relative to the polar body. (C) Oocytes had two sets of visible spindle (arrows). (D) The oocyte spindle in telophase I stage (arrow). (E) Oocytes had no visible spindle. (F) Oocyte had the abnormal spindle (arrow). (G) The oocyte spindle is with fragmentation (arrow). (H) The oocyte spindle is located under the first polar body.
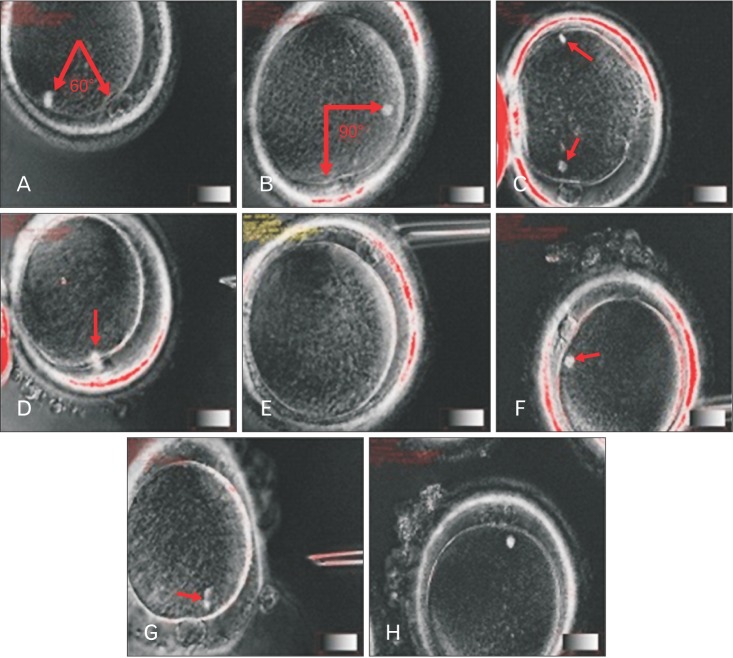
Fig. 4
Comparison of fertilization rate between spindle aligned and polar body (PB)-aligned groups (P<0.05).
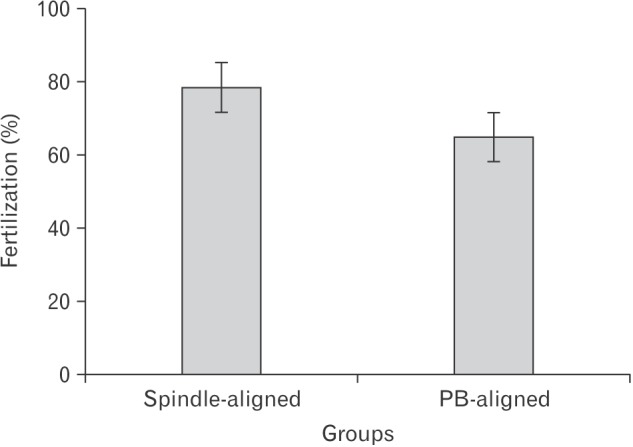
Table 1
Parameters between the control and treatment alignment groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download