Abstract
The Harris line (HL), caused by bone-growth arrest and manifesting on X-rays as a radiopaque transverse line in the metaphysis of the long bones, is an indicator reflecting stress conditions such as disease or malnutrition. HL frequency has been assumed to differ between pre-modern and modern societies, as reflective of increased caloric intake and overall nutritional improvements attendant on industrialization. To determine if such a change occurred in Korea, in the present study we compared the respective HL statuses in medieval Joseon and modern Korean population samples. HLs were found in 39.4% (28/71) of the Joseon Koreans. Whereas only 27.5% (11/40) of the males showed an HL, fully 54.8% (17/31) of the females exhibited it. Notably, HLs were observed in only 16.4% (35/213) of the modern Koreans; more remarkably still, the HL rate was almost the same between the sexes, 16.7% (20/120) for the males and 16.1% (15/93) for the females. The HL frequency was much higher in the Joseon Koreans than in their modern counterparts, reflecting the improvement of nutritional status that had been achieved in the course of South Korea's modernization. This HL-frequency decrease was much more obvious in the female populations. The higher HL frequency among the Joseon females might reflect the relatively poor nutritional condition of females in pre-modern Korean society.
In general, nutritional status of contemporary populations has been evaluated using various indicators (e.g., by means of anthropometry and medical markers such as serum albumin, prealbumin, transferrin, insulin-like growth factor-1 and total lymphocyte count) [1, 2]. However, these methods are inapplicable to studies on the nutritional status of past populations from archaeological sites. For this reason, anthropologists have focused on the measurement and interpretation of stress indicators imprinted on bones and teeth (e.g., linear enamel hypoplasia, cribra orbitalia, porotic hyperostosis, non-specific periostitis and Harris line) [3, 4].
Among these, Harris line (HL), the radiopaque transverse line detectable in the metaphysis of the long bones was first defined as an indicator of growth arrest [2]. Etiological studies have concluded that HLs can result from by nutritional stresses, especially protein and vitamin deficiencies that induce delayed development of longitudinal bone growth [3]. During endochondral bone growth, final arrest of osteoblastic activity results in the deposition of a thin layer of bone beneath the cartilage cap, which layer can become HLs [4, 5, 6, 7, 8, 9]. Subsequent recovery, which is necessary for restoration of osteoblastic activity, also is known to contribute to HL formation. When matured cartilage cells reactivate, bone growth resumes, inducing a thickened bony stratum [10]. Therefore, full recovery from periods of chronic illness or malnutrition also produces transverse lines on radiographs [10, 11]. In fact, the longer the duration and severity of malnutrition are, the thicker the HL is. HL formation in the long bones generally peaks about 2-3 years after birth. After age 5, HLs are very rare until adulthood. Also, HLs are known to occur much more frequently in boys than in girls [11].
In addition, HLs have been considered as a condition related to socioeconomic factors. HL frequency has fluctuated under various temporal and geographic conditions [12]. Especially considering the improvements in caloric intake and in overall nutritional status affected by modernization, HL frequencies in pre-modern societies may be assumed to differ from those of counterpart modern societies. Actually, HL aspects of pre-industrialization populations have already been reported for many countries [11, 13, 14, 15, 16]. However, most of these studies are limited to Europe and the Americas; and even in these, there has not been sufficient comparison studies on pre- and post-modernization changes in HL frequency.
There have been few investigations of the frequency of HL in East Asia. Moreover, few studies of general stress indicators from archaeological collections in Korea have been thus far. Accordingly, further information is required for a more detailed understanding of the general stress conditions of pre-modern populations and the transitions between pre-modern populations and their descendants in Korea. The present research aims to report the HL frequency in the medieval archaeological collection and in the modern population of Korea and to explore whether there is a statistically significant difference between the two populations. We hypothesize that if the nutritional status improved during the modernization of Korea, the HL frequency would reflect this change. Our study is based on the assumption that the presence of HL is largely caused by dietary deficiencies and illness during childhood [9, 17, 18]. To test this hypothesis, we analyzed and compared the frequencies of HL in two populations. The results of this investigation will enable us to make inferences about the life condition of the Joseon Dynasty population of Korea. Moreover, our data could provide an invaluable basis for future studies undertaken on the same subject considering that there have been few reports on HL frequency from historic East Asia.
Human tibiae (from 40 males and 31 females) collected from 16th-to-18th-century Korean tombs were examined in this study. Obviously, only tibiae for which age and sex could be confirmed by archaeological, anthropological or historical evidence were used; further, those showing severe cortical defects, fractures, tumors or other lesions were excluded from consideration.
Age was estimated correlatively to auricular-surface degeneration of the hip bone based on the degree of transverse organization, granularity, apical activity, retroauriculararea degeneration, and auricular-surface porosity [19, 20]. Accordingly, age was categorized into eight phases: 1-2, young adult (20-35 years old); 3-6, middle-aged (36-50 years old); and 7-8, old adult (over 50 years old). The right tibiae were preferentially selected for evaluation; but left tibiae alternatively were used if right tibiae were missing or unsuitable for the purposes of the study.
Antero-posterior (AP) radiographs of the Joseon tibiae, as inputted into Seoul National University Hospital (SNUH)'s Picture Archiving Communication System (PACS), were examined in the HL analysis. The radiograph parameters applied were 63 kVP, 8 mA, 11.3 milliseconds exposure time and 1.1-1.2 m distance. A modern control group was constituted of AP radiograph images obtained from 192 patients who had visited SNUH within the past five years. These images were randomly selected from within the same age and sex categories representing the Joseon samples. Radiographs revealing tibia fractures, tumors, or the other bony lesions were excluded. The radiograph parameters applied were 70 kVP, 10 mA, 20 milliseconds exposure time and 1.0 meters distance. This study was approved by the Institutional Review Board of SNUH (C-1011-036-339).
The contrast and brightness of the PACS AP radiograph images were adjusted for easier, naked-eye HL detection and examination. According to the combined criteria of several previous studies [21, 22, 23, 24], HLs were defined as follows: 1) a radiopaque transverse line extending at least 5 mm from the endosteal border to the diaphysis; 2) a distinct bone contrast extending for at least 30% or more of the tibial shaft; 3) a line forming an angle between 45 and 135 degrees obliquely to the horizontal long-bone axis, as reflective of dynamic bone remodeling [21, 24, 25]. Examples of this HL-determination methodology are shown in Fig. 1. Three raters experienced in osteology had consensus about the presence of HLs. The HL frequency was calculated by determining the ratio (n/N) of the total number of individuals with one at least or more HL (n) and total number of individuals analyzed (N). The average number of HLs was drawn by dividing the total number of HLs by the total number of the samples.
SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA) was used in the statistical analysis. Fisher's exact test was utilized to evaluate the differences of HL frequency between the Joseon and modern Koreans. An independent t-test, an ANOVA, a Mann-Whitney U test, and a Kruskal-Wallis test were used to confirm the differences in HL number. P-values less than 0.05 were considered statistically significant.
HLs were apparent in 28 of those Joseon individuals (39.4%), as compared with the only 16.4% of (35/213) modern Korean counterparts showing HLs: this represents a very significant decrease over the intervening centuries (P<0.001, chi-square test) (Table 1). As for the comparison of both sexes, whereas the frequency was higher in females (17/31, 54.8%) of Joseon Koreans than in counterpart males (11/40, 27.5%) (P=0.028, Fisher's exact test), frequencies in females (15/93, 16.1%) and the males (20/120, 16.7%) became similar for the modern Korean population (Supplementary Tables 1, 2).
When the numbers of individuals showing no, one, and two or more HLs were taken into account separately, the highest difference between Joseon and modern Koreans was observed in individuals had two or more HLs: 19.7% (14/71) of Joseon individuals and only 6.6% (14/213) of modern Koreans (P=0.003, Fisher's exact test) (Table 1). Average number of HLs in each individual, reflecting the severity of the HL (16), was also considered in this study (Table 2). Comparing the average numbers of HL for the modern and Joseon Koreans, the former was smaller than the latter (P=0.001 for total; P=0.096 for male; P=0.002 for female).
Table 3 shows the number of individuals with HLs by age group. Regarding the age groups in modern Korean people, the HL frequency in the old-adult group (10/54, 18.5%) was the highest, and the lowest was the young-adult group (5/39, 12.8%), although the difference was not statistically significant. The middle-aged group (20/120, 16.7%) was higher than young-adult group, but lower than the old-adult group. On the other hand, in the case of Joseon people, a higher HL frequency was identified for young people (53.8%) than for middle-aged (40.0%) or old people (27.8%), even if the difference was not statistically significant (Table 3). Comparing numbers of Joseon and modern individuals with HLs by age group, the frequency in young adults was higher in Joseon dynasty (53.8%) than in modern Koreans (12.8%) (P=0.005). This tendency was also observed in middle-aged group (P=0.004), but not in old-aged group (Table 3).
In Table 2, the average number of HL by age groups showed that the differences between young, middle, old age groups were not significant in each Joseon and modern group (P>0.05, Kruskal-Wallis test). However, comparing the average number of HLs of Joseon and modern Koreans by age, we found that the Joseon young adults was significantly higher than the modern counterpart (P=0.002, Mann-Whitney U test). While this phenomenon was also observed for middle-aged group (P=0.010, independent t-test), old age group showed no statistical significance (P=0.379). Finally, as for the distribution of HLs in the tibia, we found that they were more frequently observed in the distal part of tibiae than the proximal part (P<0.001) (Supplementary Table 3).
In paleopathology, HLs are considered as an indicator of episodic general stress [26, 27, 28]. Clinical and experimental studies have demonstrated that HL can result from a variety of disturbances in growth, such as nutritional deficiencies, infectious conditions, fractures, and the ingestion of certain substances (i.e., alcohol, poison) [11, 29]. For this reason, HL has received special interest as one of the few markers of health status that offer a time-depth perspective on the life of a past population [30]. However, paleopathological studies of the association between HL and other nonspecific stress markers (i.e., cribra orbitalia, porotic hyperostosis, and enamel hypoplasia) have revealed that their co-occurrence is unclear. Also, the association between HL and early age at death seems to be spurious [31] although the presence of HL is traditionally interpreted as stress markers of arrested growth in reconstructions of health status. Moreover, Alfonso-Durruty [29] suggests that HL may form not only during periods of stress as a result of growth arrest but also during normal periods of accelerated growth in which a heightened number of salutatory growth events are punctuated by an amplified number of stasis events. Accordingly, given the studies conducted over the last two decades, HL from the skeletal remains of past populations must be interpreted with caution within a cultural and demographic context [32, 33].
It is well known that long bones of young individuals have more HLs than those of older people, because youngsters are more sensitive to stress factors [16, 34]. An assessment of medieval skeletons in Southern France [14] showed that the HL number increased until the 5-to-9 age range and decreased thereafter. Hughes et al. [11] examined medieval (11th-13th century) skeletons in Ireland, finding the highest HL number among the 10-11-year-old individuals, which number was greater than that for both those up to five years old and those after the bony growth spurt. HLs are known to generally disappear with age; the rate of line disappearance depends on the bone mineralization process [21, 35].
However, our data for the modern Korean people examined did not follow the general pattern seen in the other reports. The highest HL frequency was seen in the old-adult group (10/54, 18.5%), and the lowest, in the young-adult group (5/39, 12.8%), although the difference was not statistically significant. Perhaps most notably, the HL frequency among the middle-aged group of modern Koreans (16.7%) was higher than that among the young-adult group (12.8%). We speculate that this unique pattern of HL frequency has been caused by South Korea's rapid "catch-up" industrialization of the past several decades.
As for the finding that the highest HL frequency was in the modern old-adult group, we note that Koreans over the age of 50 experienced serious hardships such as the Korean War, chronic poverty, poor hygiene, and a high prevalence of disease. This low standard of living had not markedly changed even by the 1970s: in 1973, the mortality rates (deaths per 1,000 live births) for infants and children under the age of five remained as high as 35 and 44, respectively [36].
South Korea's rapid industrialization changed everything. Between 1962 and 2011, the per-capita gross national income per capita increased almost 100-fold. As for deaths per 1,000 live births, by 2001 it had plunged to 6 for infants and 7 for children under the age of five [36]. Such a rapid and recent change in living standards almost certainly accounts for the inverse pattern of HL frequency seen among young, middle-aged and older Korean people today.
Significantly, that pattern was not observed in the Joseon samples. Rather, as is consistent with reports on other European populations, a higher HL frequency was identified for young people (53.8%) than for middle-aged (40.0%) or old people (27.8%), even if the difference was not statistically significant (Table 3).
Many of the present study's HL findings are very useful to an understanding of the stress status of Joseon people. First, the HL frequency among the Joseon samples (39.4%) was much higher than among the modern Korean subjects (16.4%). The Joseon Dynasty (1392-1910 AD) remained a relatively primitive agrarian society for which the modern safeguards and therapeutics (for example, antibiotics and effective surgical techniques) were non-existent. Naturally enough, it can be presumed that its general nutritional status was poorer than that of more modern times, which fact pushed HL frequencies among the Joseon people to relatively high levels.
We also note interesting findings concerning HL frequencies between the sexes. Among the modern Korean people we examined, HLs were observed in 16.7% of the males and 16.1% of the females, but in stark contrast, among the Joseon Koreans, there was a much lower HL frequency in the males (27.5%) than in the females (54.8%). Higher HL frequencies among females have been observed in medieval European peoples as well. For instance, there were slightly higher female HL frequencies than male in samples representing medieval Poland [16] and England [37].
We might assume that females in any pre-modern society might have been relatively more socially disadvantaged than males, for example, more exposed to stresses related to poor nutrition. This seems to have been the case for the Joseon females examined in our study, though further investigation would be necessary before any firm conclusions could be drawn. In any event, the remarkable decrease in HL frequency among the modern Korean females (16.1%) examined in our study certainly could be explained by the improvement of nutritional status achieved in the course of Korea's industrialization.
We should also note that the HL frequency for medieval Korean people was far lower than for contemporary European populations. For instance, a study on the mid-11th-to-18th-century Irish population showed an 84% HL frequency in the distal tibia, along with a 60% frequency in the proximal tibia [11]. Another study, this one by Piontek et al. [16] on medieval Polish remains, indicated a tibia HL frequency of 66.7% for males (80/120) and 72.6% for females (82/113). Besides, a study on the 8th-to-15th-century Central Switzerland adult population revealed a 74.7% (71/95) HL frequency in distal tibia [12].
This discrepancy between our medieval Korean samples and roughly contemporaneous European ones possibly could be explained by the fact that our samples do not represent the various social strata of Joseon society but mainly the upper- and ruling classes of people in the kingdom. Indeed, the tombs from which most of our samples were collected were those used by the ruling elites, which are of a type that was prohibitively expensive for people of more modest means [38]. In other words, the relatively low HL frequency found among our subjects might reflect their higher standards of living.
In conclusion, our findings revealed a considerably higher HL frequency among Joseon Koreans compared with their modern counterparts, reflecting the latter's improved nutritional standard. This decrement in HL frequency was much more obvious in females, which might reflect the poorer nutritional status of that gender in Joseon society. We also saw that the HL frequency among our medieval Korean samples was far lower than that found for other, contemporary European populations, which might be explained by the fact that most of the human skeletons examined for this study had been collected from upper-class Joseon Dynasty tombs. Since there are as yet very few HL reports on historic East Asian populations, this study should prove significant to concerned researchers.
Figures and Tables
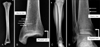
Fig. 1
An example of Harris lines (HLs) seen on the radiograph of a Joseon Korean (A, B) and modern Korean (C, D). Harris lines (thin arrows) are observed in the distal tibia. Physeal scar (thick arrows) is not counted as HL.
Acknowledgements
This work was supported by the Seoul National University Research Grant (Brain Fusion Program 2012).
References
1. Fogel RW, Engerman SL, Floud R, Friedman G, Margo RA, Sokoloff K, Steckel RH, Trussell TJ, Villaflor G, Wachter KW. Secular changes in American and British stature and nutrition. J Interdiscip Hist. 1983; 14:445–481.
2. Seres DS. Surrogate nutrition markers, malnutrition, and adequacy of nutrition support. Nutr Clin Pract. 2005; 20:308–313.
3. Robb J, Bigazzi R, Lazzarini L, Scarsini C, Sonego F. Social "status" and biological "status": a comparison of grave goods and skeletal indicators from Pontecagnano. Am J Phys Anthropol. 2001; 115:213–222.
4. Steckel RH, Rose JC, Larsen CS, Walker PL. Skeletal health in the western hemisphere from 4000 B.C. to the present. Evol Anthropol. 2002; 11:142–155.
5. Caffey J. Clinical and experimental lead poisoning: some roentgenologic and anatomic changes in growing bone. Radiology. 1931; 17:957–983.
6. Harris HA. Cessation of growth in long bones in health and disease. Mayo Clin Proc. 1927; 2:228–229.
7. Park EA, Richter CP. Transverse lines in bone: the mechanism of their development. Bull Johns Hopkins Hosp. 1953; 93:234–248.
8. Platt BS, Stewart RJ. Transverse trabeculae and osteoporosis in bones in experimental protein-calorie deficiency. Br J Nutr. 1962; 16:483–495.
9. Wolbach SB. Vitamin-A deficiency and excess in relation to skeletal growth. J Bone Joint Surg Am. 1947; 29:171–192.
10. Mays SA. The relationship between Harris line formation and bone growth and development. J Archaeol Sci. 1985; 12:207–220.
11. Hughes C, Heylings DJ, Power C. Transverse (Harris) lines in Irish archaeological remains. Am J Phys Anthropol. 1996; 101:115–131.
12. Ameen S, Staub L, Ulrich S, Vock P, Ballmer F, Anderson SE. Harris lines of the tibia across centuries: a comparison of two populations, medieval and contemporary in Central Europe. Skeletal Radiol. 2005; 34:279–284.
13. Fiscella GN, Bennike P, Lynnerup N. Transverse-Harris-lines in a skeletal population from the 1711 Danish plague site. Anthropol Anz. 2008; 66:129–138.
14. Grolleau-Raoux JL, Crubézy E, Rougé D, Brugne JF, Saunders SR. Harris lines: a study of age-associated bias in counting and interpretation. Am J Phys Anthropol. 1997; 103:209–217.
15. Gronkiewicz S, Kornafel D, Kwiatkowska B, Nowakowski D. Harris's lines versus children's living conditions in medieval Wroclaw, Poland. Var Evol. 2001; 9:45–50.
16. Piontek J, Jerszynska B, Nowak O. Harris lines in subadult and adult skeletons from the mediaeval cemetery in Cedynia, Poland. Var Evol. 2001; 9:33–43.
17. Harris HA. The growth of the long bones in childhood with special reference to certain bony striations of the methaphysis and to the role of the vitamins. Arch Intern Med. 1926; 38:785–806.
18. Acheson RM. Effects of starvation, septicaemia and chronic illness on the growth cartilage plate and metaphysis of the immature rat. J Anat. 1959; 93:123–130.
19. Lovejoy CO, Meindl RS, Pryzbeck TR, Mensforth RP. Chronological metamorphosis of the auricular surface of the ilium: a new method for the determination of adult skeletal age at death. Am J Phys Anthropol. 1985; 68:15–28.
20. Kim DK, Kim MJ, Kim YS, Oh CS, Shin DH. Vertebral osteophyte of pre-modern Korean skeletons from Joseon tombs. Anat Cell Biol. 2012; 45:274–281.
21. Garn SM, Silverman FN, Hertzog KP, Rohmann CG. Lines and bands of increased density. Their implication to growth and development. Med Radiogr Photogr. 1968; 44:58–89.
22. Gindhart PS. The frequency of appearance of transverse lines in the tibia in relation to childhood illnesses. Am J Phys Anthropol. 1969; 31:17–22.
23. Goodman AH, Clark GA. Harris lines as indicators of stress in prehistoric Illinois populations. In : Martin DL, Bumsted MP, editors. Biocultural Adaption Comprehensive Approaches to Skeletal Analysis. Research Report No. 20. Amherst: Department of Anthropology, University of Massachusetts;1981. p. 35–46.
24. Clark GA, Mack M. Reliability assessment of transverse lines. Hum Biol. 1988; 60:283–291.
25. Park EA. The imprinting of nutritional disturbances on the growing bone. Pediatrics. 1964; 33:Supple. 815–862.
26. Wells C. A new approach to ancient disease. Discovery. 1961; 22:526–531.
27. Cohen MN, Armelagos GJ. Paleopathology at the origins of agriculture. Orlando: Academic Press;1984.
28. Cunha E. Testing identification records: evidence from Coimbra identified skeletal collections (19th and 20th centuries). In : Saunders SR, Herring A, editors. Grave Reflections: Portraying the Past through Cemetery Studies. Toronto: Canadian Scholar's Press;1995. p. 179–198.
29. Alfonso-Durruty MP. Experimental assessment of nutrition and bone growth's velocity effects on Harris lines formation. Am J Phys Anthropol. 2011; 145:169–180.
30. Byers S. Technical note: calculation of age at formation of radiopaque transverse lines. Am J Phys Anthropol. 1991; 85:339–343.
31. Hatch JW, Willey PS, Hunt EE Jr. Indicators of status-related stress in Dallas society: transverse lines and cortical thickness in long bones. MidCont J Archaeol. 1983; 8:49–71.
32. Goodman AH, Martin DL. Reconstructing health profiles from skeletal remains. In : Steckel RH, Rose JC, editors. The Backbone of History: Health and Nutrition in the Western Hemisphere. New York: Cambridge University Press;2002. p. 61–93.
33. Wright LE, Yoder CJ. Recent progress in bioarchaeology: approaches to the osteological paradox. J Archaeol Res. 2003; 11:43–70.
34. McHenry H. Transverse lines in long bones of prehistoric California Indians. Am J Phys Anthropol. 1968; 29:1–17.
35. Marshall WA. Problems in relating the presence of transverse lines in the radius to the occurrence of disease. In : Brothwell DR, editor. Skeletal Biology of Earlier Human Populations. Oxford: Pergamon Press;1968. p. 241–261.
36. United Nations Population Division. World mortality report. New York: United Nations Population Division;2005.
37. Mays S. The relationship between Harris lines and other aspects of skeletal development in adults and juveniles. J Archaeol Sci. 1995; 22:511–520.
38. Shin MH, Yi YS, Bok GD, Lee EJ, Spigelman M, Park JB, Min SR, Shin DH. How did mummification occur in bodies buried in tombs with a lime soil mixture barrier during the Joseon Dynasty in Korea. In : Peña PA, Martin CR, Rodríguez MA, editors. Mummies and Science: World Mummies Research. Santa Cruz de Tenerife: Academia Canaria de la Historia;2008. p. 105–113.
Electronic Supplementary Materials
Supplementary data including three tables can be found with this article online at http://www.acbjournal.org/src/sm/acb-47-66-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download