Abstract
Therapeutic effects of various treatment options in wound healing have been one of the most controversial issues in surgical science. The present study was carried out to examine and compare the effects of Aloe vera gel, thyroid hormone cream and silver sulfadiazine cream onsutured incisions in Wistar rats. In a randomized controlled trial, thirty-six Wistar male rats, 250 to 300 g, received surgical incisions followed by topical application of Aloe vera gel, thyroid hormone cream and silver sulfadiazine 1%. To assess the efficacy of each treatment technique, a histological approach was used to evaluate the mean number of fibroblasts, macrophages, neutrophils, blood vessel sections and thickness of the regenerating epithelium and dermis on days 4, 7 and 14. Re-epithelialization and angiogenesis were significantly improved in Aloe vera gel group compared with the other treatments while thyroid hormone cream had positive effects on day 4 (P≤0.05). Topical administration of Aloe vera gel is recommended as the treatment of choice for surgical incisions.
Wound healing, a complex interaction among cells, extracellular matrix, blood vessels, proteases, cytokines, and chemokines, is still a controversial topic in surgical medicine. Despite recent improvement in understanding basic healing principles and applying a number of different strategies for wound management, there is still significant morbidity and mortality [1, 2]. Several complications such as delayed wound healing, superficial wound infection and post-operative/post-traumatic neuropathic chronic cutaneous pain have been reported by patients who had closed incisional wounds after the surgery [3, 4]. However, our knowledge about the effects of topical applications of various therapeutic options that may lead to rapid wound closure and decrease risk of infection is limited [5].
Aloe vera, an herbal plant of the lily family, has been used to treat various diseases for centuries [6]. Topical application of Aloe vera as a natural remedy, has contributed to the rapid tissue repairing process of burns, wounds, frostbite, skin infection and dermatitis [7]. Aloe vera gel (AV), which is obtained from thin-walled mucilaginous cells of the central zone of the leaf, consists of biologically active molecules that act on fibroblasts, macrophages and epidermal cells activity, stimulate formation of epidermal tissue, increase collagen synthesis and remodeling and enhance tensile stress [8].
Thyroid hormone has been recommended as a potential wound healing agent, efficient in stimulating keratinocyte proliferation, epidermal proliferation, dermal thickening and hair growth in vitro [9, 10] and in vivo [11, 12]. Thyroid therapy has been applied for the treatment of hypothyroid patients undergoing radiation-induced neck fistulae in several studies [13]. Although thyroid hormone enhances local response to growth factors and gene expression of keratins 6, 16, and 17 [14], certain studies do not support the therapeutic effects of thyroid hormone on skin wounds [15, 16].
Silver sulfadiazine (SSD) is a common anti-infective agent used to control bacterial proliferation and manage wound infection. Although indicated for deep partial-thickness and full-thickness injuries [17] and suggested as effective in reducing inflammatory and granulation phases of healing in sutured incisions [18], SSD has been reported to have cytotoxic effects on fibroblasts and keratinocytes in vitro [19] and retard wound healing in vivo [20, 21]. Despite this, SSD is the most extensively used topical agent for the treatment of wounds [22]. Little is known about the effect of SSD on the wound healing of surgically induced skin incisions [5].
In our previous study, using a biomechanical approach, we investigated and compared the effects of topical application of AV, thyroid hormone cream (TC) and SSD cream (a natural remedy, a hormone cream and an antibacterial synthetic cream) on surgically induced incisions. The results indicated that Aloe vera was significantly more effective in wound healing as attested by tensile stress parameters in sutured incisions [23]. However, to provide more evidence for our previous study, we applied a histological approach to examine and compare the effects of topical application of AV, TC and SSD cream on the healing process of sutured skin incision in Wistar rats.
Aloe vera leaves were obtained from garden of medical plants in Qeshm Island, located in the south of Iran, and sent to the Laboratory of Anatomy and Cell Biology Center, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. After washing, the base, apex and the margins were cut off to facilitate removing the transparent mucilage. A blender was used to prepare a greenish gel-like liquid. The liquid was refined using a strainer. 0.5 ml of AV was used per session.
Thirty-six Wistar male rats, 250 to 300 g, maintained in individual cages, and fed ad libitum were selected in this experiment. The study was carried out according to the "Principles of Laboratory Animal Care" (World Health Organization [WHO] Chronicle, 1985) and was approved by the Institutional Medical Ethics Committee of SBMU.
Surgical procedures were performed based on our previous study [23]. The rats were equally divided into experimental and control groups randomly (18 rats in each group), each rat in the experimental group received four incisions in the dorsolateral regions, while each rat in the control group received only one incision. All skin wounds were of 20 mm full-thickness, involving panniculus carnosus and were closed with a suture of 3-0 silk. We set the gap between the two edges of the wound at 3 mm.
The wounds in the experimental rats were treated with: AV, TC, SSD 1% (S), or V. The wounds in the control group received no medications (Fig. 1). The treatments were initiated on day 0 and were applied daily for 14 days. To investigate the process of wound healing, the animals in each group were sacrificed on days 4, 7 and 14 (6 rats on each day).
Skin tissue samples including the wound bed and normal adjacent skin, were collected after sacrificing the rats for histopathological examination purposes. These tissue samples were fixed in formaldehyde saline, embedded in paraffin wax, cut into 5 µm-thick sections and stained with hematoxylin and eosin for examination by light microscopy. We examined 10 zones from the sample morphometrically through a calibrated ocular on a light microscope (Nikon, Tokyo, Japan) at a magnification of 400× for counting fibroblasts, macrophages, neutrophils, blood vessel sections and thickness of the regenerating epithelium and dermis.
As Figs. 2 and 3 indicate the mean number of fibroblasts and blood vessel sections in AV and TC groups were significantly higher than those in V and control groups (P≤0.05). Nevertheless, no significant differences were observed among the AV and TC groups. Also these parameters were not significantly different among the S, V and control groups.
The mean number of macrophage in AV and S groups were significantly higher than that in V and control groups (P≤0.05). Nevertheless, no significant differences were observed among the AV, TC and S groups.
The mean thickness of the regenerating epithelium in AV and TC groups was significantly higher than that in other groups (P≤0.05). Nevertheless, no significant differences were observed among the AV and TC groups. Also this parameter was not significantly different among the S, V and control groups.
The mean number of neutrophils and thickness of dermis were not significantly different among the experimental and control groups.
As Figs. 4 and 5 indicate the mean number of macrophage in control group was significantly higher than that in AV and TC groups (P≤0.05). Nevertheless, no significant differences were observed among the AV and TC groups. Also this parameter was not significantly different among the S, V and control groups.
The mean number of blood vessel sections in AV group was significantly higher than that in S, V and control groups (P≤0.05). Nevertheless, no significant differences were observed among the S, V and control groups. Also this parameter was not significantly different among the AV and TC groups.
The mean of thickness of the regenerating epithelium in AV group was significantly higher than that in control group (P≤0.05). Nevertheless, no significant differences were observed among the other groups.
The mean number of fibroblasts, neutrophils and thickness of dermis were not significantly different among experimental and control groups.
The purpose of the study was to examine and compare the efficacy of topical application of AV, TC and SSD cream on the healing process of sutured incisions in Wistar rats using a histological method.
Our results showed that topical application of AV and TC can significantly increase the mean number of fibroblasts, blood vessel sections and the mean thickness of the regenerating epithelium after four days of the surgery. The mean number of macrophages also increased in AV and SSD groups on day 4. It seems the application of Aloe vera was more effective in increasing blood vessel sections and thickness of the regenerating epithelium seven days after the surgery suggesting continuing effects of this agent on wound healing. The mean number of macrophages was also significantly higher in control group as compared with Aloe vera and thyroid hormone groups which can be indicative of a delay in wound healing process in control group. Another notable point in the study was the presence of significantly higher levels of neutrophils in V and control groups 14 days after surgery suggesting a prolonged inflammatory process. The results of our histological examination provide more evidence for our previous study using a biomechanical approach in that we reported significant improvement of wound healing using AV as compared with topical applications of thyroid hormone and SSD [23]. In other words, Aloe vera can infiltrate into the skin tissue and act on the wound healing process as a whole, which is manifested as an increase in activities of biological factors involved in the repair process such as cells and enzymes as well as an improvement in blood supply and collagen content. The results support Chithra et al.'s study [8], in that Aloe vera had a beneficial impact on fibroblast proliferation, increasing in the aldehyde groups of collagen fibers, stimulation and formation of epidermal tissue and thus rapid wound closure in open wounds. The results also provide more support for those of Davis et al. [26] who confirmed the existence of mannose-6-phosphate as a major structural constituent of Aloe vera, increasing macrophage activity and stimulating fibroblast proliferation. The results are also in agreement with those of Lee et al. [27] and Moon et al. [28] confirming the angiogenesis role of Aloe vera on calf pulmonary artery endothelial cells in vitro and chick embryo membrane in vivo.
According to the results of the study, directly applied thyroid hormone has significant effect on the healing process of the incisional wounds on day 4 which provide more evidence for the other studies conducted on excisional wounds [11, 12]. Thus, it seems topical application of thyroid hormone has positive effects on wound healing mechanisms in inflammatory phase, although it can be effective in reduction of inflammatory process on day 7 and 14. Safer et al. [11, 12, 14] found that topically administered thyroid hormone was a stimulator factor on epidermal proliferation, dermal thickening, and hair growth on excisional wounds. They reported that thyroid hormone exerts influence by stimulating gene expression of keratins 6, 16, and 17 [11, 12, 14]. Redondo et al. [25] observed beneficial effects of thyroid hormone on stimulating growth of hair shafts in vitro and enhancing hair growth, proliferation of melanocytes and repigmentation of gray hair in vivo. This improvement may be related to the direct effects of thyroid hormone on thyroid hormone receptors that have been detected in fibroblasts, cells of the papillary dermis, cells of the outer root sheath and hair follicles [10].
The findings indicated that the application of SSD, as one of the most commonly applied antimicrobial agents, had no positive effect on re-epithelialization of sutured incisions supporting our previous results using biomechanical approach [23], although this agent can increase the cells involved in healing process such as macrophages in inflammatory phase. The results also showed that SSD may contribute to the decrease of inflammatory processes on day 14 as compared with V and control groups. Our results support those of Muller et al.'s study [21], in that they compared the impacts of SSD with or without Aloe vera, nystatin with and without SSD, and placebo on time to achieve 50% and 90% wound healing in excisions of Sprague-Dawley rats. No difference in acceleration of wound healing was observed between the control and SSD treatment lesions. In addition, the combination of Aloe vera with SSD has been suggested to improve wound healing [21]. Hosseinimehr et al. [29] compared the therapeutic effects of Aloe vera cream (Aloe vera powdered gel 0.5%) and SSD on burn wounds in rats reporting a significant increase in re-epithelialization when Aloe vera cream was applied. In spite of antiseptic effects of SSD on wounds, several laboratory studies confirm the cytotoxic effect of SSD on fibroblasts and keratinocytes [19, 30]. One possible explanation for this incident is debility of SSD in distinguishing between healthy cells involved in healing process and microorganisms [31].
The results of the present study indicated that Aloe vera plant gel had notable wound healing effects on sutured incisions leading to fibroblast proliferation, angiogenesis, re-epithelialization and rapid wound closure, when compared with thyroid hormone, SSD and V. However, topical administration of TC was effective in inflammatory phase of wound healing and topical administration of silver sulfadiazine cream was helpful in reduction of inflammatory processes on day 14. Therefore, AV is recommended as the treatment of choice for surgically induced incisions. Further studies comparing Aloe vera with other medications are recommended.
Figures and Tables
Fig. 2
Comparison of histological properties of incision wounds 4 days after surgery. Alphabetical letters show significant differences. Data are represented as mean±SEM. AV, Aloe vera; TC, thyroid hormone cream; SSD, silver sulfadiazine cream; V, vehicle; C, control; FB, fibroblast; MAC, macrophage; NEUT, neutrophil; VES, vessel sections; EPI, epithelial thickness; DERM, dermal thickness; mean±SEM, mean values±standard error of means of six experiments.
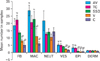
Fig. 3
Light micrographs (100×) of incisional wound bed of (A) Aloe vera group (n=6), (B) thyroid hormone cream group (n=6), (C) silver sulfadiazine cream group (n=6), (D) vehicle group (n=6), (E) control group (n=6) 4 days after surgery. Arrows depict blood vessel sections in Aloe vera and thyroid hormone cream groups. Scale bars=100 µm (A-E).
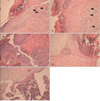
Fig. 4
Comparison of histological properties of incision wounds 7 days after surgery. Alphabetical letters show significant differences. Values are presented as mean±SEM. AV, Aloe vera; TC, thyroid hormone cream; SSD, silver sulfadiazine cream; V, vehicle; C, control; FB, fibroblast; MAC, macrophage; NEUT, neutrophil; VES, vessel sections; EPI, epithelial thickness; DERM, dermal thickness; mean±SEM, mean values±standard error of means of six experiments.
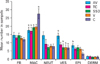
Fig. 5
Light micrographs (400×) of incisional wound bed of (A) Aloe vera group (n=6), (B) thyroid hormone cream group (n=6), (C) silver sulfadiazine cream group (n=6), (D) vehicle group (n=6), (E) control group (n=6) 7 days after surgery. Arrows depict blood vessel sections in Aloe vera and thyroid hormone cream groups. (F) Accumulation of macrophages in control group recognizably. Scale bars=20 µm (A-F).
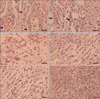
Fig. 6
Comparison of histological properties of incision wounds 14 days after surgery. Alphabetical letters show significant differences. Values are presented as mean±SEM. AV, Aloe vera; TC, thyroid hormone cream; SSD, silver sulfadiazine cream; V, vehicle; C, control; FB, fibroblast; MAC, macrophage; NEUT, neutrophil; VES, vessel sections; EPI; epithelial thickness; DERM, dermal thickness; mean±SEM, mean values±standard error of means of six experiments.
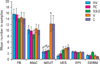
Fig. 7
Light micrographs (100×) of incisional wound bed of (A) Aloe vera group (n=6), (B) thyroid hormone cream group (n=6), (C) silver sulfadiazine cream group (n=6), (D) vehicle group (n=6), (E) control group (n=6) 14 days after surgery. Marked areas depict accumulation of neutrophils in vehicle and control groups. (F) Accumulation of neutrophils in control group with a magnification of 400×. Scale bars=100 µm (A-E), 20 µm (F).

Acknowledgements
This material is the result of work supported with resources and the use of facilities at Shahid Beheshti University of Medical Sciences.
References
1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999. 341:738–746.
2. Peacock EE, Cohen IK. McCarthy JG, May JW, Littler JW, editors. Wound healing. Plastic Surgery. 1990. Vol. 1. Philadelphia: W.B. Saunders Company;161–185.
3. Newman JT, Morgan SJ, Resende GV, Williams AE, Hammerberg EM, Dayton MR. Modality of wound closure after total knee replacement: are staples as safe as sutures? A retrospective study of 181 patients. Patient Saf Surg. 2011. 5:26.
4. Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Curr Med Res Opin. 2009. 25:2737–2743.
5. Mooney EK, Lippitt C, Friedman J. Plastic Surgery Educational Foundation DATA Committee. Silver dressings. Plast Reconstr Surg. 2006. 117:666–669.
6. Cohen SM, Rousseau ME, Robinson EH. Therapeutic use of selected herbs. Holist Nurs Pract. 2000. 14:59–68.
7. Feily A, Namazi MR. Aloe vera in dermatology: a brief review. G Ital Dermatol Venereol. 2009. 144:85–91.
8. Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998. 59:195–201.
9. Holt PJ. In vitro responses of the epidermis to triiodothyronine. J Invest Dermatol. 1978. 71:202–204.
10. Ahsan MK, Urano Y, Kato S, Oura H, Arase S. Immunohistochemical localization of thyroid hormone nuclear receptors in human hair follicles and in vitro effect of L-triiodothyronine on cultured cells of hair follicles and skin. J Med Invest. 1998. 44:179–184.
11. Safer JD, Fraser LM, Ray S, Holick MF. Topical triiodothyronine stimulates epidermal proliferation, dermal thickening, and hair growth in mice and rats. Thyroid. 2001. 11:717–724.
12. Safer JD, Crawford TM, Fraser LM, Hoa M, Ray S, Chen TC, Persons K, Holick MF. Thyroid hormone action on skin: diverging effects of topical versus intraperitoneal administration. Thyroid. 2003. 13:159–165.
13. Erdoğan M, Ilhan YS, Akkuş MA, Caboğlu SA, Ozercan I, Ilhan N, Yaman M. Effects of L-thyroxine and zinc therapy on wound healing in hypothyroid rats. Acta Chir Belg. 1999. 99:72–77.
14. Safer JD, Crawford TM, Holick MF. A role for thyroid hormone in wound healing through keratin gene expression. Endocrinology. 2004. 145:2357–2361.
15. Pirk FW, ElAttar TM, Roth GD. Effect of analogues of steroid and thyroxine hormones on wound healing in hamsters. J Periodontal Res. 1974. 9:290–297.
16. Ladenson PW, Levin AA, Ridgway EC, Daniels GH. Complications of surgery in hypothyroid patients. Am J Med. 1984. 77:261–266.
17. Ward RS, Saffle JR. Topical agents in burn and wound care. Phys Ther. 1995. 75:526–538.
18. Lansdown AB, Sampson B, Laupattarakasem P, Vuttivirojana A. Silver aids healing in the sterile skin wound: experimental studies in the laboratory rat. Br J Dermatol. 1997. 137:728–735.
19. Cooper ML, Laxer JA, Hansbrough JF. The cytotoxic effects of commonly used topical antimicrobial agents on human fibroblasts and keratinocytes. J Trauma. 1991. 31:775–782.
20. Leitch IO, Kucukcelebi A, Robson MC. Inhibition of wound contraction by topical antimicrobials. Aust N Z J Surg. 1993. 63:289–293.
21. Muller MJ, Hollyoak MA, Moaveni Z, Brown TL, Herndon DN, Heggers JP. Retardation of wound healing by silver sulfadiazine is reversed by Aloe vera and nystatin. Burns. 2003. 29:834–836.
22. Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007. 33:139–148.
23. Tarameshloo M, Norouzian M, Zarein-Dolab S, Dadpay M, Gazor R. A comparative study of the effects of topical application of Aloe vera, thyroid hormone and silver sulfadiazine on skin wounds in Wistar rats. Lab Anim Res. 2012. 28:17–21.
24. Safer JD, Crawford TM, Holick MF. Topical thyroid hormone accelerates wound healing in mice. Endocrinology. 2005. 146:4425–4430.
25. Redondo P, Guzmán M, Marquina M, Pretel M, Aguado L, Lloret P, Gorrochategui A. Repigmentation of gray hair after thyroid hormone treatment. Actas Dermosifiliogr. 2007. 98:603–610.
26. Davis RH, Kabbani JM, Maro NP. Aloe vera and wound healing. J Am Podiatr Med Assoc. 1987. 77:165–169.
27. Lee MJ, Lee OH, Yoon SH, Lee SK, Chung MH, Park YI, Sung CK, Choi JS, Kim KW. In vitro angiogenic activity of Aloe vera gel on calf pulmonary artery endothelial (CPAE) cells. Arch Pharm Res. 1998. 21:260–265.
28. Moon EJ, Lee YM, Lee OH, Lee MJ, Lee SK, Chung MH, Park YI, Sung CK, Choi JS, Kim KW. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 1999. 3:117–123.
29. Hosseinimehr SJ, Khorasani G, Azadbakht M, Zamani P, Ghasemi M, Ahmadi A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol Croat. 2010. 18:2–7.
30. Smoot EC 3rd, Kucan JO, Roth A, Mody N, Debs N. In vitro toxicity testing for antibacterials against human keratinocytes. Plast Reconstr Surg. 1991. 87:917–924.
31. Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004. 30:140–147.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download