Abstract
Stronger Neo-Minophagen C (SNMC) is a glycyrrhizin-containing preparation that is approved in Japan for the treatment of chronic hepatic diseases and is marketed in Japan, China, Korea, Taiwan, and India. Glycyrrhizin, a triterpene present in the roots and rhizomes of licorice (Glycyrrhiza glabra) has been shown to have anti-inflammatory, anti-oxidative, and anti-viral effects. In the present study, we demonstrated the marked neuroprotective effects of SNMC in the postischemic rat brain after middle cerebral artery occlusion (MCAO). We used 1 ml/kg of SNMC, which is within the dose range used for the treatment of patients with chronic hepatic disease. The administration of SNMC intravenously at 30 minutes before or 30 minutes and 3 hours after MCAO (60 minutes) reduces mean infarct volumes to 27.0±4.2%, 37.1±12.4%, and 67.8±5.8% of that of untreated controls, respectively. This neuroprotective effect is accompanied by improvements in motor impairment and neurological deficits. The administration of SNMC is shown to suppress microglia activation and neutrophil infiltration in the postischemic brain. In addition, SNMC suppresses lipopolysaccharide-induced nitrite production and proinflammatory cytokine induction in a microglia cell line, BV2. This indicates that the neuroprotective effect of SNMC might be due, at least in part, to an anti-inflammatiory effect. Interestingly, SNMC shows significantly higher neuroprotective potency compared to an equivalent dose of pure glycyrrhizin, in terms of reducing infarct volume and improving neurological deficits. Together these results indicate that SNMC, a glycyrrhizin-containing preparation developed for chronic liver disease, has a marked neuroprotective function in the postischemic brain via its anti-inflammatory effects.
Cerebral ischemia leads to brain injury via a complex series of pathophysiological events that ultimately result in neuronal death and subsequent neurological dysfunction. Excitotoxicity and Zn+2 toxicity play a key role in acute and massive neuronal death in the ischemic core [1]. This acute neuronal damage is followed by a second round of neu ronal injury in the surrounding regions, referred to as delayed neuronal death [2]. Postischemic inflammation and apoptosis, which may happen from a few hours to days after the primary ischemic event, are shown to be associated with the delayed injury [3].
Stronger Neo-Minophagen C (SNMC) is a glycyrrhizin-containing preparation that is approved in Japan for the treatment of chronic hepatic diseases and is marketed in Japan, China, Korea, Taiwan, and India [4]. It is available as a parenteral formulation (intravenous administration), and one ampoule (20 ml) contains 40 mg of glycyrrhizin, 20 mg of L-cystein, and 400 mg of glycine in a physiologic solution. Two amino acids are added to reduce the side effects of glycyrrhizin. A recent European randomized trial showed the biochemical effects of a 26-week treatment with SNMC (100 ml daily) in patients with chronic hepatitis C [5]. In addition, Arase et al. [6] demonstrated that long-term usage of SNMC (100 ml daily) is effective in preventing hepatocellular carcinoma (HCC) development in Japanese patients with chronic hepatitis C. Various mechanisms by which SNMC prevents disease progression of chronic hepatitis C have been reported.
Glycyrrhizin is present in large quantities in the roots and rhizomes of licorice (Glycyrrhiza glabra) and is composed of a molecule of glycyrrhizic acid and two molecules of glucuronic acid. This natural triterpene has been used clinically due to its anti-inflammatory, anti-allergic, and anti-viral effects [7]. Glycyrrhizin has been used in the treatment of patients with chronic hepatitis B and C [8, 9]. In addition, glycyrrhizin reduces ischemia/reperfusion-induced liver injury [10] and attenuates N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in mice and MPP+-induced cell death in PC12 cells [11]. Further, Hwang et al. [12] reported on the neuroprotective effects of roasted licorice on gerbil hippocampi after transient forebrain ischemia.
In the present study, we investigate the neuroprotective effects of SNMC in the postischemic rat brain after middle cerebral artery occlusion (MCAO), and seek to elucidate the molecular mechanism responsible for its neuroprotective effects. It is found that SNMC affords robust neuroprotection in the postischemic brain, and that these effects are, at least in part, attributable to an anti-inflammatory effect.
Male Sprague-Dawley rats (The Orient Co., Seoul, Korea) were used throughout this experiment, and randomly assigned to SNMC- and glycyrrhizin-treated or vehicle (phosphate buffered saline [PBS])-treated control groups. At the start of the experiment, animals were weighed at 280-320 g (10-week-old) and were housed separately under a 12 : 12 hour light : dark cycle with free access to food and water.
All animal experiments were carried out in accordance with "The Guidelines for Animal Research" issued by Inha University School of Medicine. MCAO is carried out as pre viously described [13]. In brief, male Sprague-Dawley rats (250-300 g) were anesthetized with 5% isoflurane in a 30% oxygen/70% nitrous oxide gas mixture; anesthesia was maintained using 0.5% isoflurane in the same gas mixture throughout the procedure. MCAO was maintained for 1 hour using a nylon suture, and this was followed by reperfusion for 12 hours to 14 days. The left femoral artery was cannulated during the procedure to obtain blood samples as well as to monitor pH, PaO2, PaCO2, and blood glucose concentration (I-STAT, Sensor Devises, Waukesha, WI, USA). Regional cere bral blood flow was monitored using a laser Doppler flowmeter (Periflux System 5000, Perimed, Jarfalla, Sweden). A thermo-regulated heating pad and heating lamp were used to maintain a rectal temperature of 37±0.5℃. Animals were randomly divided into treatment groups. Animals allocated to the sham group underwent an identical procedure to those of the other experimental group, with the exception of MCAO.
SNMC (Minophagen pharmaceutical Co., Tokyo, Japan) was administered intravenously at various doses. One ampoule (20 ml) of SNMC contains 40 mg of glycyrrhizin, 20 mg of L-cystein, and 400 mg of glycine in physiologic solution. The solution concentrations were adjusted with PBS to allow an injected volume of 0.5 ml. Glycyrhizin (Sigma, St. Louis, MO, USA) was administered intravenously in distilled water at various concentrations.
Neurological deficits were evaluated using modified Neurological Severity Scores (mNSS) at 2 day post-MCAO. The mNSS system consists of motor, sensory, balance, and re flex tests, all of which are graded using a scale of 0 to 18 (normal, 0; maximal deficit, 18) [14]. Motor scores were determined by summing the results of two tests. The first involved suspending a rat by its tail and allocating scores of zero or one to each of the following; flexion of forelimb, flexion of hindlimb, and head movement by >10° with respect to the vertical axis within 30 seconds. The second test involved placing a rat on the floor and allocating scores as follows: 0 for normal walking, 1 for an inability to walk straight, 2 for circling toward the paretic side, 3 for falling on the paretic side. Sensory tests included a placing test (score, 0-1) and a proprioceptive test (score, 0-1). A beam balance test was used to test balance and scores of 0 to 6 were allocated as follows: 0 for balanced with a steady posture, 1 for grasping the side of the beam, 2 for hugging the beam with one limb falling from the beam, 3 for hugging the beam with two limbs falling from the beam or spinning on the beam for over 60 seconds, 4 for attempting to balance on the beam but falling off within 20 to 40 seconds, 5 for attempting to balance on the beam but falling off within 20 seconds, 6 for making no attempt to balance or hang on to the beam. Reflex testing scores were determined by awarding scores to the following four items (total score, 0-4): pinna reflex, 0-1; corneal reflex, 0-1; startle reflex, 0-1; seizures, myoclonus or myodystony, 0-1.
Twenty-four hours before MCAO, rats were conditioned on a rota-rod unit at a constant 3 rpm until they were able to remain on the rotating spindle for 180 seconds. One day post-MCAO, each rat was subjected to test trial on the rota-rod at 5 rpm. Subsequently, the residence times on the rota-rod at 10 and 15 rpm were measured with 1 hour intervals between trials. This test was repeated at 2 days post-MCAO.
Rats were decapitated at 2 days post-MCAO, and their whole brains were sectioned coronally into 2-mm brain slices using a metallic brain matrix (RBM-40000, ASI, Spring ville, UT, USA). Slices were immediately stained by immersing them in 1% 2,3,5-triphenyl tetrazolium chloride at 37℃ for 15 minutes and then treating them with 4% paraformaldehyde. Infarcted areas were measured using the Scion Image program (Frederick, MD, USA). To account for cerebral edema and differential shrinkage resulting from tissue processing, the areas of ischemic lesions were adjusted using corresponding areas in ipsilateral hemispheres by subtracting the areas of ipsilateral hemispheres from those of contralateral hemispheres. Infarct volumes were calculated (in mm3) by multiplying the summed section infarct areas by section thickness.
Brains were fixed with 4% paraformaldehyde by transcar diac perfusion and post-fixed in the same solution overnight at 4℃. Brain sections (30 µm) were prepared using a vibratome, and immunological staining was performed using the method previously described [15]. The primary antibody for anti-ionized calcium binding adaptor molecule-1 (Iba-1; Wako Pure Chemicals, Osaka, Japan) was diluted to 1 : 500 and antibodies for anti-Mac2 (ABcam, Cambridge, UK) and for anti-myeloperoxidase (MPO; Dako Cytomation, Glostrup, Denmark) were diluted to 1 : 250. Subsequent to washing with PBS containing 0.1% Triton X-100, sections were incubated with anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) for anti-Mac2, or with anti-rabbit IgG (Vector Laboratories) for anti-Iba-1 in PBS for 1 hour at room temperature and visualized using the HRP/3,3'-diaminobenzidine system. All experiments were repeated at least three times and representative images are presented. Numbers of Mac2- and MPO-positive cells in 0.1 mm2 (0.32×0.32 mm) areas were obtained by counting 12 photographs, four photographs per experiment.
BV2 cells were grown in Dulbecco's modified Eagle's medium (Gibco, Carlsbad, CA, USA), supplemented with 5% fetal bovine serum (Gibco), penicillin and streptomycin (Gibco) at 37℃ in a 95% air/5% CO2 humidified atmosphere. Culture media were changed every 2 days.
BV2 cells (1×105) plated on 24-well plates were treated with lipopolysaccharide (LPS; 200 ng/ml) for 24 hours. In order to measure the amount of NO produced by microglia, 100 µl of the conditioned medium was mixed with an equal volume of Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylethylenediamine), and incubated for 10 minutes at room temperature. Absorbances were measured at 550 nm using a microplate reader.
Total RNA was prepared using a TRIzol reagent (Gibco BRL, Gaithersburg, MD, USA) and 1 µg RNA samples were used for cDNA synthesis using a RT-PCR kit (Ro che, Mannheim, Germany). Changes in the RNA levels of the proinflammatory markers, cyclooxygenase 2 (COX-2), and inducible NO syntase (iNOS), in the presence or absence of SNMC were examined at 24 hours after LPS treatment The following primers sets were used: 5'-GCTTCAAACAGTTTCTCTACAACAA-3' (forward) and 5'-CATTTCTTCCCCCAGCAAC-3' (reverse) for COX-2; 5'-AGAAGGTGGTGAAGCAGGCATC-3' (forward) and 5'-CGAAGGTGGAAGAGTGGGAGTTG-3' (reverse) for glyceraldehydes 3-phosphate dehydrogenase (GAPDH).
To investigate the neuroprotective effects of SNMC in cerebral ischemia, SNMC was administered intravenously (i.v.) at 1 ml/kg at 30 minutes before or 30 minutes, 3 hours, and 6 hours post-MCAO (60 minutes) and mean infarct volumes were assessed at 2 days post-MCAO. SNMC 1 ml/kg, which contains 2 mg/kg glycyrrhizin, is within the accepted dose range for the treatment of patients with chronic hepatic disease [5, 6]. The administrations of SNMC at 30 minutes before MCAO was shown to reduce mean infarct volumes to 27.0±4.2% of that of untreated controls (Fig. 1A, B). The administration of 1 ml/kg SNMC at 30 minutes or 3 hours post-MCAO was shown to reduce mean infarct volumes to 37.1±12.4% and 67.8±5.8% of that of untreated controls, respectively (Fig. 1A, B). These results indicate that SNMC exerts a neuroprotective effect on the postischemic brain, markedly reducing infarct volume. To compare the protective efficacy of SNMC with that of glycyrrhizin (2 mg/ml), the main ingredient of SNMC, infarct volumes of 2 or 5 mg/kg pure glycyrrhizin-treated test subjects were compared to those of subjects treated with 1 or 2.5 ml/kg of SNMC containing an equivalent amount, 2 or 5 mg/kg, of glycyrrhizin. Results indicates that SNMC reduced infarct more efficiently than the same amount of pure glycyrrhizin (Fig. 1C, D).
When SNMC (1 mg/kg) was administered at 30 minutes before or 30 minutes, 3 hours, and 6 hours post-MCAO, the mean mNSS at 2 days post-MCAO were 5.2±0.3, and 5.2±0.3, 8.5±0.6 respectively. These were significantly lower than that of the untreated MCAO group (11.0±0.5) (Fig. 2A). Motor activities were assessed using the rota-rod test at a speed of 5 rpm. Mean time spent on the rota-rod by untreated control animals at 2 days post-MCAO was 39.0±5.3 seconds (Fig. 2B), and this was markedly extended to 180.0±0, 180.0±0, and 75.3±22.3 seconds by SNMC administration at 30 minutes before or 30 minutes and 3 hours post-MCAO, respectively (Fig. 2B). In addition, scores obtained from repeated tests at 10 rpm and 15 rpm (with a 1 hour interval between tests) showed notably better motor skills for SNMC-treated animals (Fig. 2B). These results show that SNMC mitigated motor impairment and neurological deficits. As evidenced by mNSS, neurological deficits were less pronounced in SNMC (1 or 2.5 ml/kg)-treated animals (8.5±0.6 and 6.7±0.6, respectively), compared to those of an equivalent amount (2 or 5 mg/kg) of glycyrrhizin-treated animals (13.1±1.0 and 8.7±1.1, respectively) (Fig. 2C). This corroborates the results obtained from infarct volume assessment (Fig. 1C, D).
We examined whether SNMC exerts an anti-inflammatory function in the postischemic brain. Brain sections were prepared 2 days after reperfusion, and stained with antibody against Iba-1 (a marker of cells of myeloid origin) [16], Mac2 (a marker of activated resident microglia) [17], and MPO (a neutrophil marker). In sham-operated control animals, Iba-1+ cells were detected throughout the brain, in which Iba-1+ cells exhibited ramified morphology (Fig. 3A). In contrast, in the postischemic brain, Iba-1+ cells displayed a round shape with a few thick processes, indicative of the activated or phagocytic state (Fig. 3B). However, in SNMC-treated animals (1 ml/kg, 3 hours post-treatment), Iba-1+ cells displayed an inactivated (ramified) morphology similar to sham-operated animals (Fig. 3C). In contrast to Iba-1, Mac2+ and MPO+ cells were barely detected in sham-operated control animals (Fig. 3D, H). At 1 day after MCAO, Mac2+ and MPO+ cells were detected in the striatum of ischemic hemispheres (data not shown). At 2 days post-MCAO, the numbers of Mac2+ and MPO+ cells showed a marked increase in the infarction core of ischemic hemispheres (Fig. 3E, G, I, K). The number of Mac2+ cells was significantly decreased in 1 ml/kg SNMC-treated animals (Fig. 3F, G). Similarly, almost no MPO+ cells were observed in the brains of SNMC-treated animals (Fig. 3J, K). These results indicate that SNMC suppressed microglial activation and neutrophil infiltration in the postischemic brain.
To verify that the anti-inflammatory effects of SNMC is produced directly rather than indirectly, SNMC-dependent suppression of microglial activation was examined in LPS-treated BV2 cells, a microglia cell line. The cells were stimulated with LPS (0.2 µg/ml) for 24 hours with and without SNMC pre-treatment (for 1 hour) or SNMC co-treatment, and nitrite production was measured. SNMC pre-treatment reduced LPS-induced nitrite production, wherein treatment of SNMC (411.3 µl/ml), which generates 1,000 µM of glycyrrhizin concentration in the culture media, suppressed nitrite production to 29.8±7.2% of the untreated control (Fig. 4A). SNMC co-treatment reduced LPS-induced nitrite production more efficiently and in a dose-dependent manner; maximum inhibition (15.6±9.3%) was achieved with 411.3 µl/ml SNMC treatment (Fig. 4B). In addition, SNMC achieved dose-dependent repression of proinflammatory cytokine inductions, COX-2 and iNOS, in LPS-treated BV2 cells (Fig. 4C). These results indicate that SNMC suppressed the LPS-induced activation of microglial cells. In all experiments, SNMC appeared to be more efficient at such suppression that an equivalent dose of glycyrrhizin (Fig. 4).
In Japan, SNMC has been used as a treatment for chronic hepatitis for more than 30 years. In a multicenter double-blind study, alanine aminotransferase (ALT) levels in serum have been shown to significantly decrease in patients received 40 ml/day of SNMC for four weeks (P<0.001) [5, 18]. Further, when 100 ml/day SNMC was administered for eight weeks, in addition to the improved ALT levels, liver histology showed improvement in patients with chronic hepatitis, and liver cirrhosis and HCC development occurred less frequently [6, 18]. These results indicate that SNMC, in particular, a long-term treatment, prevents the development of HCC in patients with chronic hepatitis. In the present study, we examined the protective effect of SNMC in the postischemic brain at a dosage of 1 ml/kg, which is within the range of 40-80 ml of SNMC treated daily for human patients with chronic liver disease. More importantly, we administered a single bolus administration, instead of daily treatment for four or eight weeks, which strongly suggests that SNMC is indeed a potent neuroprotectant in the postischemic brain. The demonstrated marked suppression of infarct formation by 2.5 ml/kg of SNMC (Fig. 1C, D) further supports this notion.
The protective effects of SNMC have been attributed to various molecular mechanisms. The anti-inflammatory effect of SNMC was thought to be derived from the protective effect on the hepatic cellular membrane, for example, preventing lysis of hepatocytic membrane and subsequent enzyme leakages [19, 20]. Accumulating evidence points to anti-allergic, anti-inflammatory, anti-oxidative, and hepatocyte proliferation inducing effects as the underlying molecular effects of liver protection of SNMC in liver disease [21-25]. It has long been known that glycyrrhizin, a major active ingredient of SNMC, exhibits anti-oxidative effects by inhibiting 11β-hydroxysteroid dehydrogenase, which converts active corticosterone (B) to inactive 11-dehydrocorticosterone and protects the nonselective mineralocorticoid receptor from glucocorticoid excess [26]. Recently, the anti-oxidant effect of glycyrrhizin has been reported in H5N1 influenza A virus-infected cells, wherein ROS formation and redox-sensitive signaling (nuclear factor-kB [NF-kB], Jun N-terminal kinase, and p38 mitogen-activated protein kinase) are inhibited, leading to subsequent inhibition of virus replication and pro-inflammatory gene expression [4]. In the brain, NF-kB inhibition has been reported as a molecular mechanism underlying the protective effects of glycyrrhizin on N-methyl-D-aspartate (NMDA)-induced excitotoxicity in primary neurons [27].
Recently, increasing amounts of evidence indicate that binding to and inhibition of cytokine-like activity of HMGB1 is a molecular mechanism underlying the protective effects of glycyrrhizin [28]. HMGB1 acts as an endogenous danger signal, and as such, it has attracted considerable research interest. When released extracellularly, HMGB1 serves as a danger signal that evokes inflammatory reactions by activating various immune-related cells, including microglia in the case of the brain [13, 29, 30]. Intracerebral hemorrhage-induced injury is significantly attenuated by glycyrrhizin via inhibiting HMGB1 [31]. Ogiku et al. [32] reported that glycyrrhizin prevents liver injury by inhibiting HMGB1 production by Kupffer cells after ischemia-reperfusion injury. Recently, a robust neuroprotective effect of glycyrrhizin in the postischemic brain has been reported. This might be derived from the inhibition of HMGB1 secretion from activated microglia due to inhibition of HMGB1 phosphorylation via direct binding between HMGB1 and glycyrrhizin [33]. Although, the present study demonstrates the anti-inflammatory effect of SNMC, further studies regarding inhibition of HMGB1 production and secretion are necessary.
Results showed that the neuroprotective potency of SNMC was higher than the equivalent dose of pure glycyrrhizin (Figs. 1C, D, 4). This may be due to the two amino acids, cystein and glycine present in SNMC, which might function to reduce the side effects of glycyrrhizin and to stabilize it. Regarding this, Sakata et al. [34] reported that L-cystein localized in SNMC increases the reduced albumin fraction, which exerts higher anti-oxidative effects, and decreases oxidized albumin fraction, in human serum albumin preparations. The reason why SNMC is more effective than pure glycyrrhzin, including the importance of the two amino acids mentioned above, requires further study.
The remarkably efficient infarct suppression was observed when glycyrrhizin was pretreated in MCAO animal models (Fig. 1A, B). This suggests that in addition to its protracted anti-inflammatory effects, SNMC also acts to protect the brain from acute damage processes. Excitotoxicity and Zn+2-toxicity are responsible for acute and massive neuronal death in the ischemic core of the postischemic brain [1]. In this regard, the effects of SNMC in NMDA- or Zn2+-treated neurons and underlying mechanisms, including the HMGB1-dependent mechanism, need further study. Thus, it appears that the remarkable protective effects of glycyrrhizin in the postischemic brain appear to involve multiple mechanisms, which contribute to the alleviation of various aspects of brain pathology.
Figures and Tables
Fig. 1
The neuroprotective effects of glycyrrhizin in the postischemic brain. (A, B) Stronger Neo-Minophagen C (SNMC, 1 ml/kg) was administered intravenously at 30 min before or 30 min, 3 h, or 6 h after middle cerebral artery occlusion (MCAO) (60 min). (C, D) SNMC (1 or 2.5 ml/kg) or glycyrrhizin (2 or 5 mg/kg) was administered intravenously at 3 h after MCAO (60 min). (A, C) Representative images of infarctions in coronal brain sections are presented. (B, D) Mean infarction volumes were assessed at 2 days post-MCAO by 2,3,5-triphenyl tetrazolium chlorid (TTC) staining, and are presented as means±SEM (n=4-6). *P<0.05, †P<0.01.

Fig. 2
Recovery of motor deficit by Stronger Neo-Minophagen C (SNMC). (A) SNMC (1 ml/kg) was administered at 30 min before or 30 min, 3 h, or 6 h post-middle cerebral artery occlusion (MCAO) and neurological deficits were evaluated using modified neurological severity scores at 2 days post-MCAO. mNSS, modified Neurological Severity Scores. (B) The rota-rod test was performed at 5, 10 and 15 rpm at 2 days post-MCAO. (C) SNMC (1 or 2.5 ml/kg) or glycyrrhizin (2 or 5 mg/kg) was administered at 3 h after MCAO and neurological deficits were evaluated using modified neurological severity scores at 2 days post-MCAO. Sham, sham-operated group; MCAO, saline-treated MCAO group; MCAO+SNMC, SNMC-administered MCAO group, MCAO+glycyrrhizin, glycyrrhizin-administered MCAO group. Data are presented as means±SEM (n=6-9). *P<0.05, †P<0.01.
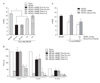
Fig. 3
Suppression of inflammation by Stronger Neo-Minophagen C (SNMC) in the postischemic brain. Brain sections were obtained 2 days after a sham procedure (A, D, H) or middle cerebral artery occlusion (MCAO) (B, C, E, F, I, J), and immunostained for ionized calcium binding adaptor molecule-1 (Iba-1) (A-C), Mac2 (D-F), or myeloperoxidase (MPO) (H-J). SNMC (1 ml/kg) was administered 3 h post-MCAO (C, F, J). Representative pictures from three independent experiments are presented. The insets in (A-J) are high magnification photographs of the indicated region (*in each photograph). (G, K) Mac2- and MPO-positive cells in regions (0.1 mm2) around indicated regions in (D-F) and (H-J), respectively, were counted and results are presented as means±SEM (n=12 from 3 animals). Scale bars=1 mm (A-F, H-J), 200 µm (A-F, H-J, inset). †P<0.01.

Fig. 4
Anti-inflammatory effects of Stronger Neo-Minophagen C (SNMC) in activated microglia. (A, B) Nitrite production was used as a surrogate of NO. BV2 cells (1×105 cells/well) were pre-treated with SNMC at the doses generating 50, 100, 250, 500, or 1,000 µM of glycyrrhizin final concentration in culture media for 1 h and then treated with lipopolysaccharide (LPS) for 24 h (A). BV2 cells (1×105 cells/well) were treated with SNMC at the doses mentioned above in (A) along with 200 ng/ml of LPS for 24 h (B). Changes in nitrite levels are presented as means±SEMs (n=4). *P<0.01. To compare the anti-inflammatory potency, 500 or 1,000 µM of pure glycyrrhizin was pretreated or co-treated with LPS and nitrite production was measured (A, B). (C) Proinflammatory cytokine production was determined by reverse transcription polymerase chain reaction 24 h after treating LPS (200 ng/ml) in the presence or absence of SNMC (generating final concentration of 250 or 500 µM glycyrrhizin in culture media) or of 500 µM of pure glycyrrhizin. iNOS, inducible NO syntase; COX-2, cyclooxygenase 2; GAPDH, glyceraldehydes 3-phosphate dehydrogenase.

Acknowledgements
This work was supported by a Research Grant from Inha University for Ja-Kyeong Lee.
References
1. Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999. 79:1431–1568.
2. Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001. 21:99–109.
3. Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982. 239:57–69.
4. Michaelis M, Geiler J, Naczk P, Sithisarn P, Leutz A, Doerr HW, Cinatl J Jr. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011. 6:e19705.
5. Orlent H, Hansen BE, Willems M, Brouwer JT, Huber R, Kullak-Ublick GA, Gerken G, Zeuzem S, Nevens F, Tielemans WC, Zondervan PE, Lagging M, Westin J, Schalm SW. Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: a randomized phase II trial. J Hepatol. 2006. 45:539–546.
6. Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997. 79:1494–1500.
7. Finney RS, Somers GF. The antiinflammatory activity of glycyrrhetinic acid and derivatives. J Pharm Pharmacol. 1958. 10:613–620.
8. Iino S, Tango T, Matsushima T, Toda G, Miyake K, Hino K, Kumada H, Yasuda K, Kuroki T, Hirayama C, Suzuki H. Therapeutic effects of stronger neo-minophagen C at different doses on chronic hepatitis and liver cirrhosis. Hepatol Res. 2001. 19:31–40.
9. Yoshida T, Abe K, Ikeda T, Matsushita T, Wake K, Sato T, Inoue H. Inhibitory effect of glycyrrhizin on lipopolysaccharide and d-galactosamine-induced mouse liver injury. Eur J Pharmacol. 2007. 576:136–142.
10. Nagai T, Egashira T, Kudo Y, Yamanaka Y, Shimada T. Attenuation of dysfunction in the ischemia-reperfused liver by glycyrrhizin. Jpn J Pharmacol. 1992. 58:209–218.
11. Kim YJ, Lee CS. Glycyrrhizin attenuates MPTP neurotoxicity in mouse and MPP-induced cell death in PC12 Cells. Korean J Physiol Pharmacol. 2008. 12:65–71.
12. Hwang IK, Lim SS, Choi KH, Yoo KY, Shin HK, Kim EJ, Yoon-Park JH, Kang TC, Kim YS, Kwon DY, Kim DW, Moon WK, Won MH. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol Sin. 2006. 27:959–965.
13. Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006. 26:6413–6421.
14. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001. 32:2682–2688.
15. Lee JK, Hwang WS, Lee YD, Han PL. Dynamic expression of SEK1 suggests multiple roles of the gene during embryogenesis and in adult brain of mice. Brain Res Mol Brain Res. 1999. 66:133–140.
16. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996. 224:855–862.
17. Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007. 27:2596–2605.
18. Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002. 62:Suppl 1. 94–100.
19. Nakamura T, Fujii T, Ichihara A. Enzyme leakage due to change of membrane permeability of primary cultured rat hepatocytes treated with various hepatotoxins and its prevention by glycyrrhizin. Cell Biol Toxicol. 1985. 1:285–295.
20. Shiki Y, Shirai K, Saito Y, Yoshida S, Mori Y, Wakashin M. Effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J Gastroenterol Hepatol. 1992. 7:12–16.
21. Park HY, Park SH, Yoon HK, Han MJ, Kim DH. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-D-glucuronide. Arch Pharm Res. 2004. 27:57–60.
22. Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000. 120:849–862.
23. Ohtsuki K, Abe Y, Shimoyama Y, Furuya T, Munakata H, Takasaki C. Separation of phospholipase A2 in Habu snake venom by glycyrrhizin (GL)-affinity column chromatography and identification of a GL-sensitive enzyme. Biol Pharm Bull. 1998. 21:574–578.
24. Kimura M, Inoue H, Hirabayashi K, Natsume H, Ogihara M. Glycyrrhizin and some analogues induce growth of primary cultured adult rat hepatocytes via epidermal growth factor receptors. Eur J Pharmacol. 2001. 431:151–161.
25. Yokozawa T, Liu ZW, Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 2000. 6:439–445.
26. Monder C, Stewart PM, Lakshmi V, Valentino R, Burt D, Edwards CR. Licorice inhibits corticosteroid 11 beta-dehydrogenase of rat kidney and liver: in vivo and in vitro studies. Endocrinology. 1989. 125:1046–1053.
27. Cherng JM, Lin HJ, Hung MS, Lin YR, Chan MH, Lin JC. Inhibition of nuclear factor kappaB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur J Pharmacol. 2006. 547:10–21.
28. Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007. 14:431–441.
29. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007. 220:35–46.
30. Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008. 28:927–938.
31. Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, Matsuoka Y, Isohama Y, Izumi Y, Kume T, Inoue A, Akaike A. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011. 61:975–980.
32. Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011. 339:93–98.
33. Kim SW, Jin YC, Shin JH, Kim ID, Park S, Han PL, Lee JK. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis. (in press).
34. Sakata M, Kawaguchi T, Taniguchi E, Abe M, Koga H, Sata M. Quick and simple method for increasing the reduced albumin fraction in human serum albumin preparations by using stronger neo-minophagen C. Hepatol Res. 2011. 41:1120–1125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download