Abstract
AMP-activated protein kinase (AMPK), an enzyme involved in energy homeostasis, regulates inflammatory responses, but its precise mechanisms are not fully understood. Recent evidence has shown that resveratrol (RES), an AMPK activator, reduces prostaglandin E2 production in lipopolysaccharide (LPS)-treated microglia. Here, we examined the effect of RES on nuclear factor kappa B (NF-κB) dependent cyclooxygenase (COX)-2 activation in LPS-treated RWA 264.7 macrophages. We found that treatment with RES increased AMPK activation. AMPK and acetyl CoA carboxylase phosphorylation were attenuated in cells treated with LPS+RES, compared to cells treated with LPS alone. RES inhibited tumor necrosis factor (TNF)-α and TNF receptor 1 in LPS-treated cells. Finally, RES inhibited LPS-induced NF-κB translocation into the nucleus and COX-2 expression. Moreover, the effects of 5-aminoimidazole-4-carboxamide ribose and compound C were consistent with the effects of RES in LPS-treated cells. Taken together, these results suggest that the anti-inflammatory action of RES in RAW 264.7 macrophages is dependent on AMPK activation and is associated with inhibition of the LPS-stimulated NF-κB-dependent COX-2 signaling pathway.
AMP-activated protein kinase (AMPK) is an energy sensor that regulates energy homeostasis and metabolic stress [1]. AMPK is activated under conditions of glucose deprivation, heat shock, oxidative stress, and ischemia [2]. Once activated, AMPK suppresses key enzymes involved in ATP-consuming anabolic pathways and increases cellular ATP supply [3]. AMPK stimulates fatty acid oxidation by phosphorylating and inhibiting acetyl CoA carboxylase (ACC) [4]. In particular, a potential role for AMPK in the suppression of inflammatory responses is supported by evidence obtained using an activator of AMPK, 5-aminoimidazole-4-carboxamide ribose (AICAR). AICAR reduces the synthesis of inducible nitric oxide synthase (NOS) by adipocytes, macrophages, and glial cells [5].
Among the immune systems that participate in host defense, macrophages are the primary cells targeted by lipopolysaccharide (LPS). LPS stimulates secretion of a variety of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6. In particular, TNF-α plays major roles in various inflammatory diseases [6, 7]. The activities of TNF are mediated by two receptors [8]. Most of the biological activities of TNF-α are mediated through TNF receptor (TNFR) 1, which plays a prominent role in anti-bacterial responses [9]. However, the role of TNFR2 is unclear.
Regulation of the nuclear factor kappa B (NF-κB) transcription factor is a key component of the TNF signaling pathway. In its inactive state, NF-κB is a cytoplasmic heterodimer that consists of three subunits: p50, p65, and IκBα. In the presence of pro-inflammatory signals, IκBα is phosphorylated and degraded via the proteasomal pathway, exposing nuclear localization signals on the p50-p65 heterodimer [10]. Cyclooxygenase (COX)-2 contributes to the pathophysiological progression of certain human cancers and inflammatory disorders [11]. The COX enzyme consists of two isoforms designated COX-1 and COX-2. COX-1 is predominantly involved in physiological and regulatory processes, whereas COX-2 is induced in a variety of healthy tissues by inflammatory cytokines, growth factors, and oncogenes [12].
Resveratrol (RES), a polyphenolic compound found in grapes and red wine, has attracted wide attention because of its antioxidant and anti-inflammatory effects [13, 14]. Numerous studies have documented the beneficial effects of RES, such as cardiovascular and cancer preventive properties [15]. Recent evidence shows that treatment with RES ameliorates elevated levels of TNF-α, IL-6, and COX-2 in experimental diabetic neuropathy [16]. RES increases AMPK activity and improves insulin sensitivity [17]. In the present study, we demonstrated that RES reduced the expression of proinflammatory mediators in LPS-treated RAW 264.7 macrophage cells in an AMPK-dependent manner.
Trans-RES was obtained from ChromaDex (Irvine, CA, USA). AICAR and compound C (CC) were obtained from Toronto Research Chemicals Inc. (Toronto, ON, Canada) and EMD4 Biosciences (Darmstadt, Germany), respectively. These reagents were dissolved in dimethylsulfoxide. LPS was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against p-AMPK, total AMPK, p-ACC, and total ACC were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against TNF-α, TNFR1 (H-5), TNFR2 (H-202), and lamin A (C-20) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibodies against NF-κB p105/p50 and COX-2 were obtained from Abcam (Cambridge, MA, USA) and Cayman Chemical Co. (Ann Arbor, MI, USA), respectively. The antibody against β-actin was purchased from Sigma-Aldrich.
RAW 264.7 macrophage cells (ATTC, Rockville, MD, USA) were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 100 U/ml streptomycin, and 2 mM glutamine at 37℃ in a 5% CO2 humidified incubator. Cells were plated at a density of 3×105 cells per 60-mm dish.
RAW 264.7 macrophages were plated at a density of 3×105 cells per 60-mm dish. The cells were rinsed with fresh medium and stimulated for 3-hours in the presence or absence of different concentrations (0.1 or 10 µM) of RES. Thereafter, RES (0.5 µM), AICAR (1 mM), and CC (10 µM) were added, and the cells were stimulated with LPS (50 ng/ml) for 3-hours.
An ELISA was performed with a TNF-α kit (R&D Systems, Minneapolis, MN, USA) to measure TNF-α concentrations in the culture supernatants, according to the manufacturer's protocol.
Cellular extracts, cytosolic fractions, and nuclear fractions were prepared according to Müller et al. [18]. The protein concentrations in each lysate were determined using a bicinchoninic acid kit (Pierce, Rockford, IL, USA), according to the manufacturer's protocol, using bovine serum albumin as the standard. Equal amounts of protein (30 µg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were washed in Tris-buffered saline containing 0.5% Tween-20 (TBST) and incubated with the following TBST-diluted primary antibodies: rabbit anti-p-AMPK (1 : 1,000), rabbit anti-total AMPK (1 : 1,000), rabbit anti-p-ACC (1 : 1,000), rabbit anti-total ACC (1 : 1,000), mouse anti-TNF-α (1 : 5,00), mouse anti-TNFR1 (1 : 500), rabbit anti-TNFR2 (1 : 500), rabbit anti-COX-2 (1 : 1,000), and rabbit anti-NF-κB p105/p50 (1 : 1,000). Samples were then incubated with their corresponding secondary antibodies. The enhanced chemiluminescence Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was used for detection. To determine the relative amounts of protein in each lane, the levels of β-actin (1 : 30,000) were used as an internal control. The quantity of nuclear proteins was determined in parallel using anti-lamin A (1 : 1,000).
Cells were cultured on cover glasses (MultiCellTM plus, CTRL Life Science, Gwangju, Korea) and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes at room temperature (RT). Permeabilization was performed in PBS with 0.3% Triton X-100 (PBS-T) for 10 minutes at RT. After blocking for 30 minutes with 5% normal donkey serum, the cells were incubated with anti-NF-κB (1 : 500) overnight at 4℃. After washing with PBS, secondary antibody (1 : 1,000) was added for 1-hour. After washing with PBS, streptavidin-AlexaFluor 594 (1 : 1,000, Invitrogen, Carlsbad, CA, USA) was added for 1-hour. Nuclei were stained with DAPI (1 : 10,000, Invitrogen), and the cells were visualized under confocal microscopy (FV-1000, Olympus, Tokyo, Japan).
We treated cells with various concentrations of RES to test the cellular toxicity of RES alone in RAW 264.7 cells (Fig. 1A). RES concentrations up to 0.5 µM were tolerated by macrophages without a significant effect on cell viability. RAW 264.7 cells were exposed to different concentrations (0.1 to 10 µM) of RES to determine AMPK expression in response to RES (Fig. 1B). Western blot analyses showed that AMPK phosphorylation in RAW 264.7 cells was enhanced significantly by treatment with RES (Fig. 1B).
We performed Western blot analyses to evaluate the effect of RES on AMPK and ACC phosphorylation in LPS-treated RAW 264.7 cells (Fig. 2). We first investigated whether RES led to AMPK activation in LPS-treated cells. As shown in Fig. 2A, the levels of p-AMPK/AMPK expression increased 3-hours after LPS treatment. However, RES attenuated LPS-induced AMPK activation in RAW 264.7 cells. Additionally, both AICAR, a AMPK activator, and CC, an AMPK inhibitor, significantly attenuated LPS-induced AMPK activation in RAW 264.7 cells (Fig. 2A). We next investigated the effect of RES on ACC phosphorylation in LPS-treated RAW 264.7 cells. In accordance with the effects on AMPK activation by RES, RES attenuated LPS-induced ACC phosphorylation in RAW 264.7 cells (Fig. 2B).
First, an ELISA was used to measure TNF-α concentrations in the supernatant of LPS-treated cells to evaluate the effect of RES on cellular TNF-α production in LPS-treated RAW 264.7 cells (Fig. 3A). TNF-α level was markedly elevated in the supernatants of LPS-treated cells. RES significantly inhibited LPS-induced TNF-α production (P<0.001). In addition, both AICAR and CC also significantly attenuated LPS-induced TNF-α expression in RAW 264.7 cells. We performed Western blot analyses to evaluate the inhibitory effect of RES on TNF-α and TNFR expression in LPS-treated RAW 264.7 cells (Fig. 3B, C). We first investigated whether RES led to inhibition of TNF-α expression in LPS-treated cells. As shown in Fig. 3A, TNF-α expression levels increased significantly 3-hours after LPS treatment. However, RES attenuated LPS-induced TNF-α expression. Moreover, both AICAR and CC significantly attenuated LPS-induced TNF-α expression in RAW 264.7 cells (Fig. 3B). We next investigated the effect of RES on TNFR1 and TNFR2 expression in LPS-treated RAW 264.7 cells. In particular, the level of TNFR1 protein was reduced by RES, as well as by AICAR and CC. However, no change in TNFR2 expression was observed in LPS-treated RAW 264.7 cells (Fig. 3C).
To confirm the effect of RES treatment on NF-κB translocation in LPS-treated RAW 264.7 cells, we performed immunocytochemistry for NF-κB, stained nuclei with DAPI, and conducted a Western blot analysis for NF-κB nuclear translocation (Fig. 4). We found nuclear co-staining for NF-κB and DAPI in LPS-treated RAW 264.7 cells but not in control cells (Fig. 4A). However, RES, AICAR, and CC treatment inhibited NF-κB translocation into the nucleus of LPS-treated RAW 264.7 cells. We further examined expression of the NF-κB p50 subunit in the cytoplasmic and nuclear extracts from each group (Fig. 4B). RES inhibited NF-κB nuclear translocation in LPS-treated RAW 264.7 cells (Fig. 4C). In addition to RES, both AICAR and CC inhibited NF-κB translocation from the cytoplasm into the nucleus.
We performed Western blot analyses for COX-2 to explore whether LPS-induced COX-2 expression in RAW 264.7 cells is altered by RES treatment (Fig. 5A). COX-2 protein expression increased significantly in LPS-treated RAW 264.7 cells. However, in accordance with the inhibitory effect of both AICAR and CC, RES significantly inhibited LPS-induced COX-2 expression 3-hours after LPS treatment (Fig. 5B).
We demonstrated that RES-induced AMPK preactivation significantly decreased pro-inflammatory cytokine TNF-α expression and NF-κB-mediated COX-2 expression in LPS-treated RAW 264.7 cells. Furthermore, treatment with an AMPK activator or inhibitor also decreased TNF-α and TNFR1-mediated NF-κB-dependent COX-2 expression in LPS-treated RAW 264.7 cells. Some reports have indicated the ability of AMPK to inhibit inflammation of macrophages, microglia, and astrocytes by inhibiting TNF-α and COX-2. In addition to the anti-inflammatory effects of AMPK activators such as RES or AICAR, the most striking finding is that CC, a direct AMPK inhibitor, exerted anti-inflammatory effects in LPS-treated RAW264.7 cells similar to those of AMPK activators. Thus, we would like to emphasize the importance of the dual effect of AMPK in macrophage-oriented inflammation.
RES is a major component of the polyphenols present in grapes and red wine. RES was originally identified as the active ingredient of an oriental herb used to treat several diseases including dermatitis, fever, hyperlipidemia, arteriosclerosis, and inflammation [19]. RES is also thought to protect against myocardial ischemia and to be a major contributor to the French paradox [13, 20]. This paradox is characterized by a lower incidence of coronary heart disease among regular drinkers of red wine, particularly in the south of France. Indeed, evidence obtained from several animal model studies suggests that RES decreases arachidonic acid release and COX-2 induction in mouse peritoneal macrophages stimulated with LPS [21].
AMPK was identified by its ability to phosphorylate and inhibit key enzymes involved in biosynthetic pathways, such as ACC (fatty acid synthesis) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (cholesterol biosynthesis) [22]. Because cholesterol metabolites attenuate inflammatory responses [23], we examined the possible role of AMPK after RES treatment in LPS-stimulated macrophages. In general, inflammatory stimuli induce the production of reactive oxygen species and increase intracellular calcium concentration [24], leading to the activation of AMPK-activating kinases [25]. As shown in Fig. 2, LPS treatment significantly increased AMPK phosphorylation; however, pretreatment with RES or AICAR significantly inhibited LPS-induced AMPK phosphorylation. Similarly, an increase in the phosphorylation of ACC, a downstream target of AMPK, was observed and was a good correlate of AMPK activation. Furthermore, some reports have been contradictory about the effect of AMPK on anti-apoptotic activity or cell death. Long-term stimulation of AMPK with AICAR prevents apoptosis in astrocytes [26]. However, despite the possible protective effects, several reports have shown that AMPK promotes cell death in a variety of cell types, including neurons, and that inhibiting AMPK protects cells from ATP depletion-induced cell death following exposure to various stressors [27, 28]. In the present study, we identified AMPK as a potent counter-regulator of macrophage inflammatory function. Our data show that RES concentrations up to 0.5 µM were tolerated by macrophages without a significant effect on cell viability. AMPK expression increased significantly with 0.5 µM RES. This result indicates that RES pretreatment provides a preconditioning state for a decrease in the ATP to AMP ratio and then counteracts the LPS-activated AMPK phosphorylation in macrophage cells. In contrast, sustained AMPK activation may aggravate inflammation after cellular stresses or insults. We also found that CC may have an ambivalent effect on the inflammatory response in LPS-treated RAW 264.7 macrophages as an AMPK inhibitor. Thus, we suggest that modest preactivation of AMPK by RES or inhibition of AMPK by CC may induce positive effects such as an anti-inflammatory effect.
TNF-α is a potent proinflammatory cytokine released primarily from stimulated macrophages. Many aspects of tissue injury following acute or chronic inflammatory responses can be directly attributed to the concomitant induction of TNF biosynthesis and release [29]. All known responses to TNF are triggered by binding to one of two distinct receptors, TNFR1 or TNFR2, which are differentially regulated on various cell types in normal and diseased tissues [30]. Based on cell culture and animal studies of each receptor in knockout mice, proinflammatory and cell death pathways, which are activated by TNF, are mediated primarily through TNFR1 [31]. The effect of TNFR2 signaling is less characterized. In the present study, we clearly demonstrated that RES-induced AMPK preactivation can be mediated by inhibiting TNF-α through TNFR1 expression caused by LPS stimulation. This result is consistent with previous evidence that AICAR suppresses LPS-induced TNF-α secretion in RAW 264.7 cells [32]. Thus, our data indicate that RES may inhibit inflammatory mediators by preactivating AMPK.
The most striking finding of our study was that CC, a direct AMPK inhibitor, had anti-inflammatory properties similar to those of RES and AICAR. In particular, we demonstrated that CC attenuated TNF-α protein expression in LPS-treated RAW 264.7 cells to an equivalent or greater extent than RES or AICAR. This result is consistent with a previous study showing that CC down-regulates IL-1β, IL-6, and TNF-α release in LPS-stimulated microglia [33]. However, it does not support their observation that CC did not alter the p-AMPK/AMPK ratio in either LPS-stimulated or non-stimulated microglia. A recent study demonstrated that the inhibitory effect of berberine on iNOS and COX-2 expression was abolished by CC-induced AMPK inhibition [34]. This supports our result that CC reduced levels of TNF-α and COX-2 expression in LPS-stimulated RAW 264.7 cells. Our results showed that the p-AMPK/AMPK ratio in LPS+CC-treated cells decreased significantly compared that in LPS+RES-treated cells (Fig. 2A). This result indicates that CC could act as direct AMPK inhibitor and counteract LPS-induced AMPK activation. In particular, we demonstrated that both activators and inhibitors of AMPK attenuated LPS-induced AMPK phosphorylation in RAW 264.7 cells. Our results may suggest that an AMPK inhibitor could act as a direct AMPK inhibitor. In contrast, AMPK activators could offer a preventive condition against insult-induced changes in cellular energy homeostasis and counteract LPS-induced AMPK activation. Thus, we suggest that modest preactivation of AMPK may contribute to anti-inflammatory properties similar to those of an AMPK inhibitor. Although pretreatment with CC reduced LPS-induced ACC phosphorylation, the degree of reduction in ACC phosphorylation was different compared to AMPK phosphorylation. This difference indicates that the relative expression level of AMPK or ACC phosphorylation in LPS-treated cells was different compared to that in controls.
Several groups have suggested that AMPK serves as an anti-inflammatory target. For example, AMPK activation by AICAR downregulates LPS-mediated induction of the proinflammatory cytokine iNOS and NO production in rat glial cells by inhibiting NF-κB nuclear translocation [5]. Metformin also attenuates monocyte infiltration in an experimental model of multiple sclerosis [35]. In fact, the NF-κB cascade is considered one of the major players in the inflammatory response. NF-κB activation results in elevated levels of inflammatory mediators. COX-2 activity has also been correlated with inflammatory damage. In experimental diabetic neuropathy, RES treatment attenuates elevated levels of p65, IκB-α, and COX-2 in diabetic rats and protects against diabetic neuropathy [16]. Recent reports have shown that berberine activates AMPK in adipocytes, endothelial cells, macrophages, and the liver [36, 37]. Berberine has been used to treat type 2 diabetes in China [38]. The anti-inflammatory effect of berberine in BV-2 microglia is consistent with our results, indicating that RES suppresses inflammation responses through AMPK activation in LPS-stimulated macrophages.
In conclusion, our results demonstrate that RES-induced AMPK preactivation had a therapeutic effect in LPS-treated RAW 264.7 cells, and that this effect was mediated by activating the AMPK/ACC signaling pathway. In particular, we demonstrated that RES-induced AMPK preactivation decreased rather than increased LPS-induced AMPK expression in RAW 264.7 cells. Our results show that RES significantly attenuated TNF-α-mediated TNFR1 expression, NF-κB translocation, and COX-2, similar to the anti-inflammatory effects of AICAR. In addition to the anti-inflammatory effects of AMPK activators such as RES or AICAR, the most striking finding was that CC, a direct AMPK inhibitor, had anti-inflammatory properties in LPS-treated RAW264.7 cells similar to those of AMPK activators. Thus, our results indicate that RES may play an important role in anti-inflammation, and new targets for the AMPK/ACC signaling pathway could be used as effective therapies for inflammatory diseases such as diabetes mellitus and neurodegenerative diseases.
Figures and Tables
Fig. 1
Effect of resveratrol (RES) treatment on AMP-activated protein kinase (AMPK) activation in RAW 264.7 cells. (A) Concentration-dependent effects of RES on RAW 264.7 cell viability. Cells were incubated with different concentrations (0.1 or 10 µM) of RES for 3-h. (B) Western blot analysis for AMPK activation in different concentrations of RES. p-AMPK was normalized to β-actin and represented as arbitrary units. Data are expressed as mean±SEM of the ratio of p-AMPK protein expression for three independent experiments. P<0.05 by ANOVA. *P<0.005,†P<0.001 vs. control.

Fig. 2
Effect of resveratrol (RES) treatment on AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) phosphorylation in lipopolysaccharide (LPS)-treated RAW 264.7 cells. Cells were incubated with 0.5 µM RES for 3-h, 1 mM 5-aminoimidazole-4-carboxamide ribose (AICAR) for 1-h, and 10 µM compound C (CC) for 1-h. Cells were then treated with 50 ng/ml LPS for 3-h. Cell lysates were subjected to Western blot analysis with p-AMPK and total AMPK (A) and p-ACC and total ACC (B) antibodies. p-AMPK or p-ACC was normalized to total AMPK or ACC and represented as arbitrary units, respectively. Data are expressed as mean±SEM for three independent experiments. P<0.05 by ANOVA. *P<0.005, †P<0.001 vs. control (CTL) or LPS-treated cells, respectively.

Fig. 3
Effect of resveratrol (RES) treatment on tumor necrosis factor (TNF)-α and TNF receptor (TNFR) expression in lipopolysaccharide (LPS)-treated RAW 264.7 cells. Cells were incubated with 0.5 µM RES for 3-h, 1 mM 5-aminoimidazole-4-carboxamide ribose (AICAR) for 1-h, and 10 µM compound C (CC) for 1-h. Cells were then treated with 50 ng/ml LPS for 3-h. (A) Cell supernatant TNF-α concentrations measured by enzyme-linked immunosorbent assay. Cell lysates were subjected to Western blot analyses with TNF-α (B) and TNFR (C) antibodies, respectively. TNF-α and TNFR1 were normalized to β-actin and represented as arbitrary units. Data are expressed as mean±SEM for three independent experiments. P<0.05 by ANOVA. *P<0.005, †P<0.001 vs. the respective control (CTL) or LPS-treated cells, respectively.

Fig. 4
Effect of resveratrol (RES) treatment on nuclear factor κB (NF-κB) translocation in lipopolysaccharide (LPS)-treated RAW 264.7 cells. (A) Representative photomicrographs of immunolabeling for NF-κB (red) and nuclear counterstaining with DAPI (blue) in control (CTL), LPS, LPS+RES, LPS+5-aminoimidazole-4-carboxamide ribose (AICAR), and LPS+compound C (CC)-treated RAW 264.7 cells. LPS treatment induced increased colocalization of NF-κB and DAPI (purple) in LPS-treated cells. However, RES treatment reduced NF-κB translocation, as shown by decreased colocalization with DAPI. Arrows indicate translocation from the cytoplasm to the nucleus. Scale bar=100 µm. (B) Nuclear translocation of cytoplasmic NF-κB from each group was assessed by Western blotting. (C) Quantitative analysis of NF-κB levels calculated from Western blots. Cytoplasmic (cyto) or nuclear (nu) NF-κB was normalized to β-actin or lamin A and represented as arbitrary units, respectively. Data are expressed as mean±SEM for three independent experiments. P<0.05 by ANOVA. *P<0.05, †P<0.001 vs. the respective control (CTL) or LPS-treated cells, respectively.
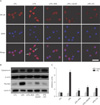
Fig. 5
Effect of resveratrol (RES) treatment on cyclooxygenase (COX)-2 expression in lipopolysaccharide (LPS)-treated RAW 264.7 cells. (A) Cells were incubated with 0.5 µM RES for 3-h, 1 mM 5-aminoimidazole-4-carboxamide ribose (AICAR) for 1-h, and 10 µM compound C (CC) for 1-h. Cells were then treated with 50 ng/ml LPS for 3-h. Cell lysates were subjected to Western blot analysis with a COX-2 antibody. (B) Quantitative analysis of COX-2 levels calculated from Western blots. COX-2 was normalized to β-actin and represented as arbitrary units. Data are expressed as mean±SEM for three independent experiments. P<0.05 by ANOVA. *P<0.001 vs. the respective control (CTL) or LPS-treated cells, respectively.

Acknowledgements
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korea government (MEST) (No. 2009-0064327).
References
1. Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006. 116:1776–1783.
2. Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994. 4:315–324.
3. Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999. 24:22–25.
4. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002. 30(Pt 6):1064–1070.
5. Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004. 24:479–487.
6. Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002. 277:22131–22139.
7. Raabe T, Bukrinsky M, Currie RA. Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem. 1998. 273:974–980.
8. Wajant H, Scheurich P. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int J Biochem Cell Biol. 2001. 33:19–32.
9. Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998. 95:570–575.
10. Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003. 24:1269–1279.
11. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001. 2:149–156.
12. Ermert L, Ermert M, Goppelt-Struebe M, Walmrath D, Grimminger F, Steudel W, Ghofrani HA, Homberger C, Duncker H, Seeger W. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol. 1998. 18:479–488.
13. Frémont L. Biological effects of resveratrol. Life Sci. 2000. 66:663–673.
14. Miller NJ, Rice-Evans CA. Antioxidant activity of resveratrol in red wine. Clin Chem. 1995. 41(12 Pt 1):1789.
15. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.
16. Kumar A, Sharma SS. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010. 394:360–365.
17. Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010. 59:551–553.
18. Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993. 187:233–256.
19. Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull (Tokyo). 1982. 30:1766–1770.
20. Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, Bertelli AA, Bertelli A, Das DK. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000. 35:263–268.
21. Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999. 126:673–680.
22. Hardie DG. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992. 1123:231–238.
23. Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997. 100:2671–2679.
24. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007. 10:1387–1394.
25. Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006. 203:1665–1670.
26. Blázquez C, Geelen MJ, Velasco G, Guzmán M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001. 489:149–153.
27. McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005. 280:20493–20502.
28. Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005. 25:9554–9575.
29. Tracey KJ, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993. 21:10 Suppl. S415–S422.
30. Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest. 2001. 81:1503–1515.
31. Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008. 214:149–160.
32. Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004. 318:372–380.
33. Labuzek K, Liber S, Gabryel B, Bułdak L, Okopień B. Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures. Naunyn Schmiedebergs Arch Pharmacol. 2010. 381:41–57.
34. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010. 110:697–705.
35. Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009. 182:8005–8014.
36. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009. 296:E955–E964.
37. Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009. 82:484–492.
38. Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009. 606:262–268.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download