Abstract
We observed how the hypothyroid state affects diabetic states and modifies cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus (DG). For this, 0.03% methimazole, an anti-thyroid drug, was administered to 7-week-old, pre-diabetic Zucker diabetic fatty (ZDF) rats by drinking water for 5 weeks, and the animals were sacrificed at 12 weeks of age. At this age, corticosterone levels were significantly increased in the ZDF rats compared to those in the control (Zucker lean control, ZLC) rats. Methimazole (methi) treatment in the ZDF rats (ZDF-methi rats) significantly decreased corticosterone levels and diabetes-induced hypertrophy of adrenal glands. In the DG, Ki67 (a marker for cell proliferation)- and doublecortin (DCX, a marker for neuronal progenitors)-immunoreactive cells were much lower in the ZDF rats than those in the ZLC rats. However, in ZDF-methi rats, numbers of Ki67- and DCX-immunoreactive cells were similar to those in the ZLC rats. These suggest that methi significantly reduces diabetes-induced hypertrophy of the adrenal gland and alleviates the diabetes-induced reduction of cell proliferation and neuronal progenitors in the DG.
Thyroid hormones regulate developmental processes such as neurogenesis, myelination, dendrite proliferation and synapse formation (Bernal et al., 2003; Williams, 2008). In particular, maternally synthesized thyroid hormones at very late embryonic stages influence neuronal proliferation and migration of neurons in the cerebral cortex, hippocampus and medial ganglionic eminence (Narayanan & Narayanan, 1985; Ausó et al., 2004; Cuevas et al., 2005). In addition, a close association exists between thyroid hormones and brain cholinergic function (Smith et al., 2002). These effects are mainly observed in specific cholinergic nuclei and their pathways, such as the basal forebrain and the hippocampus (Patel et al., 1987).
Hippocampal neurons are vulnerable to diabetes (Gispen & Biessels, 2000; Magariños & McEwen, 2000); memory loss and impaired executive function also accompany type 2 diabetes (Ryan & Geckle, 2000). In addition, diabetes reduces neuroblasts in the dentate gyrus of the hippocampus in type 1 (Jackson-Guilford et al., 2000; Beauquis et al., 2006) and type 2 (Hwang et al., 2008) models; these neuroblasts extend their axons and contact CA3 pyramidal neurons in the hippocampus proper, becoming integrated into the hippocampal circuitry (Stanfield & Trice, 1988; Hastings & Gould, 1999). In diabetic rats, hyper-activation of the hypothalamo-pituitary adrenal axis is well described, and corticosterone in the adrenal gland mediates diabetes-induced impairments of hippocampal synaptic plasticity and neurogenesis, as well as associated cognitive deficits (Landfield et al., 1978; Trudeau et al., 2004; Montaron et al., 2006; Stranahan et al., 2008). The correlation between thyroid hormones and adrenal corticosteroids hormones has been reported (Silva & Bianco, 2008).
Although there are reports about the effects of hypothyroidism in the type 1 (Hibbe et al., 1991) and type 2 diabetic models (Matsushita et al., 2005; Tamura et al., 2005; Hwang et al., 2009), no studies have been reported about the effects of hypothyroidism in type 2 diabetic model on cell proliferation and neuroblast differentiation. In the present study, we investigated the consequences of adult-onset hypothyroidism in diabetic rats using methimazole, an anti-thyroid drug, which has been used in the management of hyperthyroid patients (Cooper 2005). We also investigated how the hypothyroid state modifies neuroblast differentiation which retarded by diabetic state possibly through high corticosterone level in the hippocampal dentate gyrus of Zucker diabetic fatty (fa/fa, ZDF) rats by measuring expression of Ki67, an endogenous marker of proliferation expressed during late G1, S, M and G2 phases of cell cycle (Cooper-Kuhn & Kuhn, 2002), and doublecortin (DCX), a marker of neuronal progenitors differentiating into neurons (Karl et al., 2005).
Male and female Zucker diabetic heterozygote rats (fa/+) were purchased from Genetic Models (Indianapolis, IN, USA) and mated each other. They were housed in a conventional state under adequate temperature (23℃) and humidity (60%) control with a 12-h light/12-h dark cycle, and free access to food and water. Purina 5008 rodent diets (7.5% fat) were provided as recommended by Genetic Models. The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Genotype of fa gene herein was determined with the strategy described previous our study (Hwang et al., 2008, 2009). ZDF rats were randomly divided into 2 groups (n=7 per group) with vehicle-ZDF and hypothyroid-ZDF group. At 7 weeks of age, hypothyroidism was induced by the administration of 0.03% 2-mercapto-1-methyl-imidazole (methimazole, Sigma, St. Louis, MO, USA) in drinking water for 5 weeks. ZLC rats (n=7) were served as the control. All animals were euthanized at 12 weeks of age.
To measure blood glucose concentration, blood was analyzed by using a blood glucose monitor (Ascensia Elite XL Blood Glucose Meter, Bayer, Toronto, ON, Canada). To confirm the hypothyroid state and corticosterone levels, the animals were anesthetized with 60 mg/kg chloral hydrate and blood specimens were drawn from the right ventricle of ZLC, ZDF and methimazole-treated ZDF (ZDF-methi) rats at 12 weeks of age. After collection, the blood samples were centrifuged (5 min, 14,000 r.p.m., 4℃) and serum samples were stored in liquid nitrogen until measurement. Serum T4 and corticosterone were measured using commercially available RIA kits from Monobind Incorporation (CA, USA) and IBL (Germany), respectively.
For histological staining, ZLC, ZDF and ZDF-methi rats were perfused by a previous mentioned method (Hwang et al., 2008, 2009). In brief, adrenal glands were dehydrated with graded concentrations of alcohol for embedding in paraffin. Thereafter paraffin-embedded tissues were sectioned on a microtome (Leica, Wetzlar, Germany) into 3-µm coronal sections, and they were mounted into silane-coated slides. The sections were stained with hematoxylin and eosin (H&E) according to general protocol.
For immunohistochemistry, brains were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica) into 30 µm coronal sections and then the sections were collected into six-well plates containing PBS. Immunohistochemistry was performed under the same conditions in each group in order to examine whether the degree of immunohistochemical staining was accurate. Sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal goat or rabbit serum in 0.05 M PBS for 30 min. They were then incubated with diluted rabbit anti-Ki67 (1 : 1,000, Abcam, Cambridge, UK) or goat anti-DCX antibody (1 : 50, SantaCruz Biotechnology, Santa Cruz, CA, USA) overnight at room temperature and subsequently exposed to biotinylated goat anti-rabbit IgG or rabbit anti-goat IgG and streptavidin peroxidase complex (diluted 1 : 200, Vector, Burlingame, CA, USA). They were then visualized by staining with 3,3'-diaminobenzidine in 0.1 M Tris-HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were mounted in Canada Balsam (Kanto, Tokyo, Japan) following dehydration.
All measurements were performed in order to ensure objectivity in blind conditions, by two observers for each experiment, carrying out the measures of control and experimental samples under the same conditions.
For quantitative analysis of the number of Ki67 or DCX positive cells in the hippocampus, 15 section with 60 µm interval were selected from each animals according to anatomical landmarks corresponding to Bregma -3.00~-4.08 mm of rat brain atlas (Paxinos & Watson, 2007). The corresponding areas of the hippocampus were measured on the monitor at a magnification of 100×. Images of Ki67 or DCX-immunoreactive cells taken from dentate gyrus were obtained through a BX51 light microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DP71, Olympus) connected to a PC monitor. The number of Ki67 or DCX positive cells in dentate gyrus was analyzed by Optimas 6.5 software (CyberMetrics, Scottsdale, AZ). In addition, dendritic complexity of DCX positive cells was analyzed using the accompanying software (NeuroExplore, MicroBrightField, Inc., VT,), calculating complexity including dendritic length and number of branches. Cell counts were obtained by averaging the counts from the sections taken from each animal: A ratio of the count was calibrated as %.
The GraphPad Prism (Ver 4.03) statistical analysis software was used for all data analysis. The data shown here represent the means of experiments performed for each experimental area. Differences among the means were statistically analyzed by one-way ANOVA test followed by Duncan's new multiple range method.
At 12 weeks of age, blood glucose levels were reported by our previous study (Hwang et al., 2009). Serum T4 levels in ZLC rats were 76.3 µg/dL. In ZDF rats, T4 levels were significantly increased to 10.31 µg/dL. In ZDF-methi rats, T4 levels were significantly decreased by 62% compared to that in the ZDF group. In this group, T4 levels were 3.95 µg/dL (Fig. 1A).
Serum corticosterone level in ZLC rats was 218.9 ng/mL. In ZDF rats, corticosterone level was significantly higher (461.7 ng/mL) than that in ZLC rats. In ZDF-methi rats, corticosterone level was significantly decreased (289.3 ng/mL) compared to that in ZDF rats (Fig. 1B).
In ZLC, ZDF and ZDF-methi rats, morphologies of adrenal glands were differently found. First of all, the size of the adrenal medulla was markedly different among these groups. In ZDF rats, the adrenal medulla and the zona fasciculata of the adrenal cortex were significantly enlarged, but they were significantly decreased in ZDF-methi rats. Indeed, in the ZDF-methi rats, the size of adrenal gland was somewhat smaller than that in ZLC rats (Fig. 2A~F).
In ZLC rats, some Ki67-immunoreactive nuclei were detected in the subgranular zone of the dentate gyrus (Fig. 3A). However, in ZDF rats, Ki67-immunoreactive nuclei were significantly decreased by 33.4% compared to those in ZLC rats (Fig. 3B and D). In ZDF-methi rats, Ki67-immunoreactive nuclei were significantly increased compared to those in ZDF rats (Fig. 3C). In this group, the number of Ki67-immunoreactive nuclei was slightly lower than that in ZLC rats (88.2% vs. ZLC rats) (Fig. 3D).
In ZLC rats, DCX-immunoreactive cells were detected in the subgranular zone of the dentate gyrus (Fig. 4A) and DCX had well-developed (tertiary) dendrites, which were extended into two-thirds of molecular layer of the dentate gyrus (Fig. 4B). In the ZDF rats, DCX-immunoreactive neuroblasts were significantly decreased in the dentate gyrus and fewer DCX-immunoreactive neuroblasts with tertiary dendrites were detected (Fig. 4C, D and G). In ZDF-methi rats, the number of DCX-immunoreactive neuroblasts with tertiary dendrites was increased compared to that in the ZDF rats and were similar to that in ZLC rats (Fig. 4E, F and G).
The ZDF rat is a well-characterized genetic model of non-insulin-dependent (type 2) diabetes and obesity. ZDF rats have a defective leptin receptor (Zucker & Zucker, 1961) and diabetes typically appears between 7 and 10 weeks of age and is maintained for at least 6 months (Etgen & Oldham, 2000).
In this study, we observed that treatment with methimazole in ZDF rats decreased the serum blood glucose levels. In addition, methimazole treatment in ZDF rats significantly reduced the hypertrophy of adrenal medulla and zona fasciculata of adrenal cortex and serum corticosterone levels. These results are supported by previous studies that hypothyroidism in rats resulted in decreased adrenal weights and plasma concentrations of corticosterone (Tohei et al., 1991 and 1998; Tohei 2004).
We observed, in the present study, effects of methimazole on cell proliferation and neuronal differentiation in the subgranular zone of the hippocampal dentate gyrus in adult type 2 diabetic rats, because neurogenesis in the hippocampal dentate gyrus is associated with cognitive performance (Song et al., 2002; Siwak-Tapp et al., 2007; Aizawa et al., 2009). Ki67- and DCX-immunoreactive cells were markedly low in the dentate gyrus in ZDF rats, however, in methimazole treatment in ZDF rats, numbers of Ki67- and DCX-immunoreactive cells were similar to those ZLC rats.
Indeed, hippocampal neurogenesis is significantly decreased in diabetic animals (Jackson-Guilford et al., 2000; Beauquis et al., 2006; Hwang et al., 2008) and diabetic patients (Trudeau et al., 2004). Methimazole has dual actions on memory functions in healthy and diabetic rats. Hypothyroidism is also reported to impair cognition and memory in adult patients and animal models (Mennemeier et al., 1993; Wilcoxon et al., 2007) and to impair long-term potentiation in the rat hippocampus (Lee et al., 2003). In addition, thyroid dysfunction has been shown to influence acetylcholinesterase activity in both developing and adult rats (Carageorgiou et al., 2007). Furthermore, hypothyroidism reduces 5-bromo-deoxyuridine-positive cells (Ambrogini et al., 2005) and DCX-immunoreactive neuroblasts, which exhibit severely hypoplastic dendritic arborization (Montero-Pedrazuela et al., 2006). However, in ZDF rats, hypothyroidism alleviated the diabetic phenotypes and diabetes-induced reduction of DCX-immunoreactive cells. This effect may be associated with hypothyroidism-induced reduction of serum corticosterone levels. Treatment with antisense oligonucleotides directed against glucocorticoid receptor has been reported to restore normal fasting glucose levels in Zucker diabetic rats (Watts et al., 2005). In addition, lowering corticosterone prevented diabetes-induced impairment of learning and memory in insulin-deficient rats and insulin-resistant (db/db) mice (Stranahan et al., 2008). It has also been reported that corticosterone levels began to increase as a consequence of aging (Landfield et al., 1978), and lowering corticosterone from mid-age protected from the age-related decline in hippocampal neurogenesis and cognitive functions (Montaron et al., 2006). In addition, hypothyroidism induced by methimazole resulted in a significant decrease in the plasma concentrations of corticosterone (Weng et al., 2007), which has negative correlation with neurogenesis (Joëls 2007).
Exposure to elevated corticosterone reduces insulin receptor signaling in the brain (Piroli et al., 2007), and finally, the negative effect of diabetes on hippocampal plasticity may be attributable to an interaction between elevated glucocorticoids and insulin receptor signaling. In the present study, lowering corticosterone levels significantly increased Ki67- and DCX-immunoreactive cells in the dentate gyrus. This result is supported by a previous study that lowering corticosterone levels in diabetes could restore behavioral functions (Stranahan et al., 2008).
In conclusion, treatment with methimazole in ZDF rats, type 2 diabetic rats, significantly alleviated increases of serum corticosterone levels and enlargement of the adrenal gland. In addition, methimazole rescued the diabetes-induced reduction of Ki67- and DCX-immunoreactive cells. The reduction of diabetic phenotypes and rescue of cell proliferation and neuronal differentiation may be associated with hypothyroidism-related reduction of corticosterone.
Figures and Tables
Fig. 1
T4 (A) and corticosterone levels (B) in ZLC, vehicle-treated ZDF and methimazole-treated ZDF (ZDF-methi) rats at 12 weeks of age. Serum T4 and corticosterone levels are significantly high in ZDF rats compared to that in the ZLC rats. In ZDF-methi rats, serum corticosterone levels are lower than that in ZDF rats. The bars indicate means±SE (n=7 per group; *P<0.05, significantly different from ZLC rats, †P<0.05, significantly different from ZDF rats).

Fig. 2
Hematoxylin and eosin staining of the adrenal gland in ZLC (A and D), vehicle-treated ZDF (B and E) and methimazole-treated ZDF (ZDF-methi) rats (C and F) at 12 weeks of age. The size of adrenal gland in zona fasciculata (zf) of cortex and adrenal medulla is significantly decreased in the ZDF-methi rats compared to that in the ZDF rats and slightly smaller than that in ZLC rats. zg, zona glomerulosa; zr, zona reticulata. Bar = 400 µm (A~C), 100 µm (D~F).
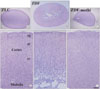
Fig. 3
Ki67 immunohistochemistry in the dentate gyrus in ZLC (A) and vehicle-treated ZDF (B) and methimazole-treated ZDF (ZDF-methi) (C) rats. Ki67 immunoreaction is detected in the subgranular zone of the polymorphic layer (PoL). Ki67-immunoreactive cells are significantly increased in ZDF-methi rats compared to those in the ZDF rats. GCL, Granule cell layer; ML, Molecular layer. Bar=100 µm. (D) Mean number of Ki67-immunoreactive cells per section in the dentate gyrus of the ZLC, ZDF and ZDF-methi rats (n=7 per group; *P<0.05, significantly different from the ZLC rats, †P<0.05, significantly different from the ZDF rats). The bars indicate means±SEM.

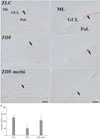
Fig. 4
DCX immunohistochemistry in the dentate gyrus in ZLC (A and B), vehicle-treated ZDF (C and D) and methimazole-treated ZDF (ZDF-methi) (E and F) rats. DCX immunoreaction is detected in the subgranular zone (arrows) of the polymorphic layer (PoL). DCX-immunoreactive cells are significantly increased in ZDF-methi rats compared to those in the ZDF rats. GCL,Granule cell layer; ML, Molecular layer. Bar=100 µm (A, C, and E), 50 µm (B, D, and F). (G) Mean number of DCX-immunoreactive neuroblasts with/without tertiary dendrites per section in the dentate gyrus of ZLC, ZDF and ZDF-methi rats (n=7 per group; *P<0.05, significantly different from the ZLC rats, †P<0.05, significantly different from ZDF the rats). The bars indicate the means±SEM.
Acknowledgements
The authors would like to thank Mr. Seung Uk Lee and Mrs. Hyun Sook Kim for their technical help in this study. This study was supported by the Regional Core Research Program funded by the Korea Ministry of Education, Science and Technology (Medical & Bio-material Research Center).
References
1. Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009. 58:403–407.
2. Ambrogini P, Cuppini R, Ferri P, et al. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005. 81:244–253.
3. Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004. 145:4037–4047.
4. Beauquis J, Roig P, Homo-Delarche F, De Nicola A, Saravia F. Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: reversion by antidepressant treatment. Eur J Neurosci. 2006. 23:1539–1546.
5. Bernal J, Guadaño-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003. 13:1005–1012.
6. Carageorgiou H, Pantos C, Zarros A, et al. Changes in acetylcholinesterase, Na+,K+-ATPase, and Mg2+-ATPase activities in the frontal cortex and the hippocampus of hyper- and hypothyroid adult rats. Metabolism. 2007. 56:1104–1110.
7. Cooper DS. Antithyroid drugs. N Engl J Med. 2005. 352:905–917.
8. Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002. 134:13–21.
9. Cuevas E, Ausó E, Telefont M, Morreale de Escobar G, Sotelo C, Berbel P. Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur J Neurosci. 2005. 22:541–551.
10. Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 2000. 49:684–688.
11. Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000. 23:542–549.
12. Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999. 413:146–154.
13. Hibbe T, Kiesel U, Kolb-Bachofen V, Kolb H. Methimazole treatment aggravates low-dose streptozotocin-induced diabetes. Diabetes Res Clin Pract. 1991. 11:53–58.
14. Hwang IK, Kim IY, Kim YN, et al. Effects of methimazole on the onset of type 2 diabetes in leptin receptor-deficient rats. J Vet Med Sci. 2009. 71:275–280.
15. Hwang IK, Yi SS, Kim YN, et al. Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2008. 33:394–400.
16. Jackson-Guilford J, Leander JD, Nisenbaum LK. The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett. 2000. 293:91–94.
17. Joëls M. Role of corticosteroid hormones in the dentate gyrus. Prog Brain Res. 2007. 163:355–370.
18. Karl C, Couillard-Despres S, Prang P, et al. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem. 2005. 92:264–282.
19. Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978. 202:1098–1102.
20. Lee PR, Brady D, Koenig JI. Thyroid hormone regulation of N-methyl-D-aspartic acid receptor subunit mRNA expression in adult brain. J Neuroendocrinol. 2003. 15:87–92.
21. Magariños AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA. 2000. 97:11056–11061.
22. Matsushita M, Tamura K, Osada S, Kogo H. Effect of troglitazone on the excess testosterone and LH secretion in thyroidectomized, insulin-resistant, type 2 diabetic Goto-Kakizaki rats. Endocrine. 2005. 27:301–305.
23. Mennemeier M, Garner RD, Heilman KM. Memory, mood and measurement in hypothyroidism. J Clin Exp Neuropsychol. 1993. 15:822–831.
24. Montaron MF, Drapeau E, Dupret D, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006. 27:645–654.
25. Montero-Pedrazuela A, Venero C, Lavado-Autric R, et al. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006. 11:361–671.
26. Narayanan CH, Narayanan Y. Cell formation in the motor nucleus and mesencephalic nucleus of the trigeminal nerve of rats made hypothyroid by propylthiouracil. Exp Brain Res. 1985. 59:257–266.
27. Patel AJ, Hayashi M, Hunt A. Selective persistent reduction in choline acetyltransferase activity in basal forebrain of the rat after thyroid deficiency during early life. Brain Res. 1987. 422:182–185.
28. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2007. Amsterdam: Elsevier Academic Press.
29. Piroli GG, Grillo CA, Reznikov LR, et al. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007. 85:71–80.
30. Ryan CM, Geckle MO. Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care. 2000. 23:1486–1493.
31. Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid. 2008. 18:157–165.
32. Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007. 88:249–259.
33. Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002. 26:45–60.
34. Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002. 5:438–445.
35. Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988. 72:399–406.
36. Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008. 11:309–317.
37. Tamura K, Osada S, Matsushita M, Abe K, Kogo H. Changes in ovarian steroidogenesis in insulin-resistant, type 2 diabetic Goto-Kakizaki rats after thyroidectomy and gonadotropin treatment. Eur J Pharmacol. 2005. 513:151–157.
38. Tohei A. Studies on the functional relationship between thyroid, adrenal and gonadal hormones. J Reprod Dev. 2004. 50:9–20.
39. Tohei A, Akai M, Tomabechi T, Mamada M, Taya K. Adrenal and gonadal function in hypothyroid adult male rats. J Endocrinol. 1997. 152:147–154.
40. Tohei A, Imai A, Watanabe G, Taya K. Influence of thiouracil-induced hypothyroidism on adrenal and gonadal functions in adult female rats. J Vet Med Sci. 1998. 60:439–446.
41. Trudeau F, Gagnon S, Massicotte G. Hippocampal synaptic plasticity and glutamate receptor regulation: influences of diabetes mellitus. Eur J Pharmacol. 2004. 490:177–186.
42. Watts LM, Manchem VP, Leedom TA, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005. 54:1846–1853.
43. Weng Q, Saita E, Watanabe G, et al. Effect of methimazole-induced hypothyroidism on adrenal and gonadal functions in male Japanese quail (Coturnix japonica). J Reprod Dev. 2007. 53:1335–1341.
44. Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007. 177:109–116.
45. Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008. 20:784–794.
46. Zucker LM, Zucker TF. Fatty, a new mutation in the rat. J Hered. 1961. 52:275–278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download