1. Kim JS, Cha JK, Cho AR, Kim MS, Lee JS, Hong JY, et al. Acceleration of bone regeneration by BMP-2-loaded collagenated biphasic calcium phosphate in rabbit sinus. Clin Implant Dent Relat Res. 2015; 17:1103–1113.

2. LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003; 14:201–209.

3. Lim HC, Zhang ML, Lee JS, Jung UW, Choi SH. Effect of different hydroxyapatite:beta-tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int J Oral Maxillofac Implants. 2015; 30:65–72.

4. Mangano C, Sinjari B, Shibli JA, Mangano F, Hamisch S, Piattelli A, et al. A human clinical, histological, histomorphometrical, and radiographical study on biphasic HA-beta-TCP 30/70 in maxillary sinus augmentation. Clin Implant Dent Relat Res. 2015; 17:610–618.
5. Choi Y, Lee JS, Kim YJ, Kim MS, Choi SH, Cho KS, et al. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: a histometric analysis in rabbits. Tissue Eng Part A. 2013; 19:1994–2004.
6. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002; 13:405–409.

7. Brunner E, Langer F. Nonparametric analysis of ordered categorical data in designs with longitudinal observations and small sample sizes. Biom J. 2000; 42:663–675.

8. Torrecillas-Martínez L, Galindo-Moreno P, Ávila-Ortiz G, Ortega-Oller I, Monje A, Hernández-Cortés P, et al. Significance of the immunohistochemical expression of bone morphogenetic protein-4 in bone maturation after maxillary sinus grafting in humans. Clin Implant Dent Relat Res. 2016; 18:717–724.

9. Hong JY, Kim MS, Lim HC, Lee JS, Choi SH, Jung UW. A high concentration of recombinant human bone morphogenetic protein-2 induces low-efficacy bone regeneration in sinus augmentation: a histomorphometric analysis in rabbits. Clin Oral Implants Res. Forthcoming. 2015.

10. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002; 27:2662–2673.

11. Yon J, Lee JS, Lim HC, Kim MS, Hong JY, Choi SH, et al. Pre-clinical evaluation of the osteogenic potential of bone morphogenetic protein-2 loaded onto a particulate porcine bone biomaterial. J Clin Periodontol. 2015; 42:81–88.

12. Kim MS, Kwon JY, Lee JS, Song JS, Choi SH, Jung UW. Low-dose recombinant human bone morphogenetic protein-2 to enhance the osteogenic potential of the Schneiderian membrane in the early healing phase:
in vitro and
in vivo studies. J Oral Maxillofac Surg. 2014; 72:1480–1494.

13. Kenley RA, Yim K, Abrams J, Ron E, Turek T, Marden LJ, et al. Biotechnology and bone graft substitutes. Pharm Res. 1993; 10:1393–1401.

14. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009; 31:1825–1835.

15. Jung RE, Weber FE, Thoma DS, Ehrbar M, Cochran DL, Hämmerle CH. Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydroxyapatite/tricalciumphosphate. Clin Oral Implants Res. 2008; 19:188–195.

16. Kim JW, Jung IH, Lee KI, Jung UW, Kim CS, Choi SH, et al. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012; 100:3304–3313.

17. Jang JW, Yun JH, Lee KI, Jang JW, Jung UW, Kim CS, et al. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:480–487.

18. Lim HC, Hong JY, Lee JS, Jung UW, Choi SH. Late-term healing in an augmented sinus with different ratios of biphasic calcium phosphate: a pilot study using a rabbit sinus model. J Periodontal Implant Sci. 2016; 46:57–69.

19. Kim MS, Lee JS, Shin HK, Kim JS, Yun JH, Cho KS. Prospective randomized, controlled trial of sinus grafting using Escherichia-coli-produced rhBMP-2 with a biphasic calcium phosphate carrier compared to deproteinized bovine bone. Clin Oral Implants Res. 2015; 26:1361–1368.

20. Park JC, Kim JC, Kim BK, Cho KS, Im GI, Kim BS, et al. Dose- and time-dependent effects of recombinant human bone morphogenetic protein-2 on the osteogenic and adipogenic potentials of alveolar bone-derived stromal CELLS. J Periodontal Res. 2012; 47:645–654.

21. Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res. 2012; 23:682–689.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



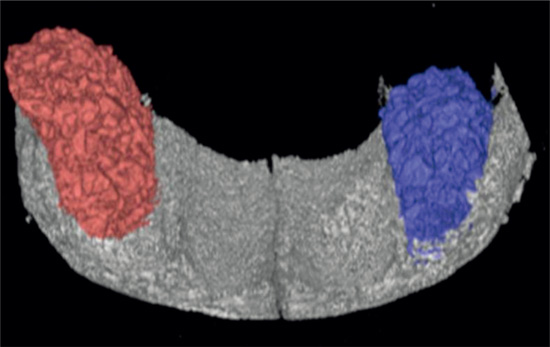
 XML Download
XML Download