Abstract
Purpose
In this study, the effect of calcium sodium phosphosilicate (NovaMin) desensitizing agent, which is a powder-based system, and hydroxyethyl methacrylate and glutaraldehyde (Gluma desensitizer), which is liquid-based system, on dentinal tubule occlusion was analyzed by scanning electron microscope. The effects of the above two along with one control group were compared to determine the more effective method of sealing the dentinal tubules after initial application.
Methods
Twenty specimens were allocated to each of 3 groups: Control, Gluma desensitizer, and NovaMin. Two additional samples were also prepared and treated with Gluma and NovaMin; these samples were longitudinally fractured. The specimens were prepared from extracted sound human premolars and were stored in 10% formalin at room temperature. The teeth were cleaned of gross debris and then sectioned to provide one to two dentin specimens. The dentin specimens were etched with 6% citric acid for 2 minutes and rinsed in distilled water. Control discs were dried, and the test discs were treated with the desensitizing agents as per the manufacturer's instructions. The discs as well as longitudinal sections were later analyzed under the scanning electron microscope. The proportions of completely occluded, partially occluded, and open tubules within each group were calculated. The ratios of completely and partially occluded tubules to the total tubules for all the groups was determined, and the data was statistically analyzed using nonparametric tests and statistical significance was calculated.
Following the decline of dental caries, the management of periodontal diseases gained priority, and other, painful dental problems, such as dentin hypersensitivity drew attention [1]. In 1982, dentin hypersensitivity was described as an enigma because it was frequently encountered yet poorly understood [2].
Dentin hypersensitivity is described clinically as an exaggerated response to a nonnoxious sensory stimulus, such as osmotic changes, thermal changes, or mechanical stimuli. It is viewed as originating from the underlying exposed dentin after the enamel or cementum at the root surface has been eroded away [3]. Loss of enamel or tooth structure occurs by attrition, abrasion, or erosion, whereas denudation of the root surface can occur as a result of gingival recession, periodontal therapy, or improper tooth brushing [4]. In general, a slightly higher incidence of dentin hypersensitivity is reported in females than in males, and in those aged from 20 to 40 years. However, the peak occurrence is found at the end of the third decade, and is most commonly reported from the buccal cervical zones of permanent teeth. Sites of predilection in descending order are the canines and first premolars, incisors and second premolars, and molars [5].
It has been shown by Brannstrom in human studies that the patency of the dentinal tubules is a major characteristic of sensitive dentin. A significant positive correlation between the density of open dentinal tubules and the intensity of pain responses induced from exposed cervical dentin surfaces has also been reported. The condition of dentin with either open or blocked tubules is decisive regarding the hydraulic conductance of dentin and thus stimulus-induced fluid flow in the dentinal tubules. Hence, blocking of the tubules should abolish dentinal pain symptoms effectively [6].
Thus, there are two principal treatment options, either to plug the dentinal tubules, preventing fluid flow, or desensitizing the nerve, making it less responsive to stimulation [7]. There is a vast array of treatment available for desensitization including solutions, gels, and pastes that contain fluorides in varying compounds and percentages, calcium hydroxide, strontium chloride, potassium nitrate, sodium citrate, glutaraldehyde and hydroxyethyl methacrylate, potassium, or ferric oxalate. A combination product consisting of an aqueous solution of 5% glutaraldehyde and 35% hydroxyethyl methacrylate (Gluma desensitizer, Heraeus Kulzer GmbH, Wehreim, Germany) has been reported to be an effective desensitizing agent. The glutaraldehyde intrinsically blocks dentinal tubules, counteracting the hydrodynamic mechanism that leads to dentin hypersensitivity [8]. A new product consisting of calcium sodium phosphosilicate (NovaMin, DenShield, Alachua, FL, USA) has been introduced. NovaMin is a trade name that has been given to bioactive glass (e.g., Bioglass) that has been ground into a fine particulate with a median size of less than 20 microns. It reduces sensitivity by blocking open tubules and by supplying calcium (Ca2+) and phosphate (PO43-) ions when the environment is optimum to form hydroxycarbonate apatite (HCA). It is composed of elements that are naturally occurring in the body and reacts to form a mineral layer that is chemically and structurally similar to natural tooth material [9].
The major objective of this study was to evaluate and compare the effect of Gluma desensitizer, a liquid-based system, and NovaMin, a powder-based system, on dentinal tubule occlusion after their initial application as desensitizing agents in the treatment of dentinal hypersensitivity.
In this study, extracted sound human premolars were included, all of which had been extracted for orthodontic reasons and had no history of scaling, root planing, or prophylaxis in the previous six months. The dentin specimens were prepared from these extracted sound human premolars and were stored in 10% formalin at room temperature.
The teeth were cleaned of gross debris, and a total of 60 dentin samples were prepared. All tooth cuts were made with a carborundum disc attached to a cutting machine. The crown and the apical third of each tooth were removed, and the remaining teeth were sectioned to provide one to two dentin specimens each. Sectioned samples of 2-mm thickness were made. The dentin specimens were then placed in an ultrasonic cleaner in distilled water for 30 seconds, etched with 6% citric acid for 2 minutes to remove the smear layer and rinsed in distilled water. The control specimens were then dried, and the test specimens were treated with the desensitizing agents as per the manufacturer's instructions. Out of all the sections, a total of 60 specimens were taken. These specimens were named sample I (Figs. 1-3). The specimens from sample I were then randomly assigned to 3 groups of 20 specimens each. The control group was surface treated with distilled water, the second group, with Gluma desensitizer, and the third group with NovaMin.
In the Gluma group, a small amount of Gluma desensitizer, which comes as a liquid in a small bottle, was applied onto the dentin discs using small cotton pellets as per the manufacturer's instructions and left for 30-40 seconds. The surface was then dried by applying a stream of compressed air until the fluid film had disappeared and the surface was no longer shiny, and then rinsed thoroughly with water. In the NovaMin group, a few drops of water were added to the NovaMin, which comes as a powder in a vial, to form a paste according to the manufacturer's instructions, and then it was applied to the dentin specimens with the help of a swab. It was left for 2 minutes and then lightly rinsed away. The specimens were then dried in a desiccator.
In addition to the above mentioned samples, two more samples were prepared in a manner similar to the previous samples. These two samples were treated with Gluma desensitizer and NovaMin, respectively. The two samples were than fractured with the help of an orthodontic cutter to obtain the longitudinal section of the specimen. These specimens were analyzed to observe the penetration of the materials into the tubules and notice any changes in the surface of the dentin. These specimens were named sample II (Figs. 4 and 5).
The samples were mounted on the small stub with the help of silver paste. These samples were placed in an ionsputtering device. Under a high vacuum, ions are discharged from the gold target to the cathode (i.e., the samples). The specimens were sputter coated with a thin layer of gold in a vacuum using a fine coat ion sputter (JFC-1100, JEOL, Tokyo, Japan). This ensured a proper conduction surface to the non-conducting specimens. Ions were sputtered on the samples for 5 minutes and thus the samples were ready for the scanning electron microscope (SEM).
The sample I specimens were then examined by one SEM (JSM 840 A, JEOL), and the sample II specimens were examined by another SEM (Leo-440i-SEM, Leo electron microscopy, Cambridge, UK).
Photographs of the samples were obtained from the camera, which was fixed to the SEM. The surface of sample I specimens were scanned and observed on the fluorescent screen at a magnification of ×3,000, and the photographs of the representative areas were obtained (Figs. 1-3). The total number of tubules, number of open, number of completely occluded, and number of partially occluded tubules were counted in each photograph of all of the specimens. The specimens of sample II were scanned and analyzed at magnification of ×5,000, ×10,000, and ×20,000, and the images of the corresponding areas were obtained (Figs. 4 and 5).
The following criteria was used for determining the type of occlusion when counting the tubules. The tubules that showed complete penetration of the crystal or complete obliteration of the canals with the reaction products were considered completely occluded. Those that showed reduction of the diameter of the tubule by more than fifty percent or circumferential closure of the tubule with the presence of a central opening in the canal were considered partially occluded.
The total number of tubules was counted from the various images captured by the SEM. Out of the total tubules, those that were completely occluded, partially occluded, and open tubules were counted. The ratio of completely occluded tubules to the total tubules as well as the ratio of partially occluded tubules to the total tubules were calculated. The data obtained did not show a normal distribution; therefore, nonparametric tests were done. The data obtained was statistically analyzed using the Kruskal-Wallis test and Wilcoxon rank sum test, through which comparison among the groups as well as intergroup comparison was performed, respectively, and statistical significance was calculated (Table 1). The mean of the ratio of completely occluded tubules to total tubules as well as partially occluded to total tubules for each group were plotted (Figs. 6 and 7). All of the statistical analyses were performed by using IBM SPSS ver. 21 (IBM Co., Armonk, NY, USA).
SEM studies of hypersensitive dentin surfaces reveal that they have more patent tubules per unit area than nonsensitive dentin. Furthermore, tubules in superficial parts of hypersensitive dentin are on average twice as wide as tubules in nonsensitive dentin. Absi et al. [10] and Yoshiyama et al. [11] reported that in naturally desensitized dentin, most of the tubules were occluded. On the basis of transmission electron microscopic studies, Yoshiyama et al. [11] reported that tubular occlusions could be due to extension of the intratubular dentin layer or deposition of substances in the tubules. Some of the occlusions in their study were crystals of inorganic salts, but some may be organic in origin. However, the nature of the occluding layer is important. Some surfaces where the tubules were observed to be occluded with a "dense pellicle" were found to be very sensitive. Pashley and Carvalho [12] noted that tubules apparently occluded with a smear plug are permeable to both solvent and solute. Thus, the surface appearance alone may not correlate with sensitivity or permeability [8].
The width of the tubule is very important, as the rate of fluid flow is dependent on the fourth power of the radius. If the tubule diameter doubles, a 16-fold increase in fluid flow results. Sensitive teeth have many more (8×) and wider (2×) tubules at the buccal cervical area compared with nonsensitive teeth. A higher velocity of fluid flow also occurs in tubules of smaller diameter, possibly provoking pain sensations. Dentin will only be sensitive if the tubules are patent from the pulp to the oral environment, and this patency will change with production and removal of the smear, hence resulting in an episode condition [13]. Most studies on tubule occlusion have focused on coronal dentin, where important variables such as the dentin surface area, thickness, and surface characteristics can be controlled. The validity of data collected in vitro, however, is open to criticism. The hydraulic conductance of radicular dentin has been observed to be much lower than that of coronal dentin; there is a good correlation between tubule density and diameter and the measured hydraulic conductance [14].
One problem highlighted in the Morris et al. [15] study was the very powerful placebo effect inherent in clinical dentin sensitivity studies, particularly when dealing with small numbers of subjects and eligible teeth. Furthermore, the large standard deviations reported by Morris et al. [15], because of the highly subjective nature of pain and/or the variability of the individual pain response reported in dentin sensitivity studies, makes it extremely difficult to detect significant differences between groups without utilizing a large number of subjects. With this in mind, the in vitro examination of products using a reproducible model such as the dentin disc, can aid the understanding of the potential occluding, and thus desensitizing properties of possible desensitizing agents [16].
Gluma desensitizer is an aqueous solution containing 5% glutaraldehyde and 35% hydroxyethyl methacrylate. Because glutaraldehyde is a biological fixative, it has been suggested that the dentinal tubules are occluded as an effect of reaction with plasma proteins from dentinal fluid. Hydroxyethyl methacrylate is a hydrophilic monomer compound of dentin bonding agents with the ability to infiltrate into acid-etched and moist dental hard tissue [17].
NovaMin is a material that has been recently introduced and has been shown to reduce sensitivity by blocking open tubules in both in vitro and in vivo studies (United States patents 5,735,942 and 6,086,374) [18,19]. NovaMin is a bioactive glass-ceramic material that falls into a class of newer agents that provide calcium and phosphate upon reaction. In the case of products with NovaMin, the active ingredient is a calcium sodium phosphosilicate that reacts when exposed to aqueous media and provides calcium and phosphate ions that form a HCA with time [20]. The combination of the residual NovaMin particles and the HCA layer results in the physical occlusion of dentinal tubules, which will relieve hypersensitivity [21].
In our study, most of the tubules in the control group were found to be open (Fig. 1), with some of them occluded with a smear layer; on the other hand, most of the tubules in the sections treated with Gluma (Fig. 2) and NovaMin (Fig. 3), which work on the principle of tubule occlusion by infiltration of precipitation products, were partially or completely occluded. In our study, after initial application, Gluma desensitizer produced a greater number of partially occluded tubules and fewer completely occluded tubules, while in the case of specimens treated with NovaMin, a greater number of completely occluded tubules and fewer partially occluded tubules were observed; in both the cases, the difference was statistically significant. This might be due to the mechanism of Gluma desensitizer, which reportedly is based on total or partial closure of the tubules by protein coagulation and precipitation upon reaction with glutaraldehyde and hydroxyethyl methacrylate [15]. The study of longitudinal sections by SEM micrograph is one way of understanding the interaction of applied material on the treated surface. Specimens treated with Gluma desensitizer showed a resinous layer of thickness 1-2 µm occluding the surface of the tubules and characteristically showed transverse septa in the lumen of the dentinal tubules as a result of glutaraldehyde action [22,23] (Fig. 4). On the other hand, specimens treated with NovaMin showed a 2- to 3-µm-thick layer with large crystalline particles occluding the tubules in some regions. The tubules were occluded with the crystal-like precipitates (Fig. 5). A study by Litkowski [9] also substantiates the findings in support of NovaMin, which demonstrate in vivo relief from application of NovaMin after 2 weeks, and on SEM analysis, it was confirmed that it caused complete occlusion, which can be credited for relief from hypersensitivity. Results of both the groups were, however, statistically significantly different from those of the control group. An in vitro study reported that NovaMin occluded a significantly greater number of dentinal tubules relative to untreated controls, and it also occluded significantly more tubules than another test material, Quell desensitizer [24]. The results of the current study revealed that NovaMin-treated dentin specimens showed more complete tubule occlusion. This is in accordance with the findings of Litkowski [9] and Du Min et al. [25], who found NovaMin to be a more effective desensitizer. An in vivo study reported that Gluma desensitizer was not effective in relieving dentinal hypersensitivity after 4 weeks; this can be attributed to a relatively large number of open and partially occluded tubules remaining after treatment [26].
In the present study, we have shown that professionally applied dental (in-office) products containing NovaMin (calcium sodium phosphosilicate) and Gluma desensitizer are both capable of occluding the dentin tubules to varying degrees and may have the clinical potential to reduce dentin hypersensitivity. Both desensitizers occluded the tubules but NovaMin has shown superior results in terms of complete tubule occlusion on initial application. The results of the present study are limited to physical findings of the change in the dentinal tubules, and do not present in vivo differences that may result from the physiological effect of these desensitizing agents. Differences between our results and those of other studies may be related to the dentin specimen utilized, etching process, time and mode of application of the desensitizing agent, or a combination of these variables. Significant differences in results can be produced on multiple applications and testing the materials under the vigorous conditions. In this study, it has been shown that NovaMin and Gluma desensitizer are materials with different modes of action and produce varying degrees of obliteration of tubules at initial application and hence could have differences in reduction in sensitivity based on the type and amount of blockage of tubules. Both materials produced varying degrees of tubule occlusion in the form of complete and partial occlusion.
In conclusion, NovaMin was found to produce more completely occluded tubules while Gluma desensitizer caused more partial occlusion on initial application. There was a statistically significant difference between the two groups when the ratio of complete and partial occlusion was calculated against the total number of tubules. Hence, NovaMin application could be more effective in providing relief from dentinal hypersensitivity.
Figures and Tables
Figure 1
Morphology of dentinal tubules treated with distilled water (control), seen under scanning electron microscope (×3,000).

Figure 2
Morphology of dentinal tubules treated with Gluma desensitizer, seen under scanning electron microscope (×3,000).

Figure 3
Morphology of dentinal tubules treated with NovaMin, seen under scanning electron microscope (×3,000).

Figure 4
(A, B) Longitudinal sections of a specimen treated with Gluma desensitizer at ×20,000 and ×10,000 respectively. The arrows show characteristic transverse septa while the circled area shows an occluded area.
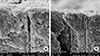
Figure 5
(A, B) Longitudinal sections of a specimen treated with NovaMin at ×5,000. The arrows show large hydroxycarbonate apatite particles, while circled area shows an occluded area.
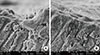
Figure 6
Ratio of completely occluded tubules to total tubules. The bar graph depicts the mean ratio of the number of completely occluded tubules to the total number of tubules. The mean value is highest for the NovaMin group, which indicates more completely occluded tubules than the other groups.

Figure 7
Ratio of partially occluded tubules to total tubules. The bar graph depicts the mean ratio of the number of partially occluded tubules to the total number of tubules. The mean value is highest for the Gluma desensitizer group, which indicates more partially occluded tubules than the other groups.

Table 1
Inter and multiple group comparison as well as mean and stardard deviation calculation of control, Gluma, and NovaMin
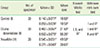
As the data was not normally distributed nonparametric Kruskal-Wallis test was used to compare all three groups while another nonparametric Wilcoxon rank-sum test was used for comparison of each group with the other.
SD: standard deviation.
a)Ratio of completely occluded and total tubules. b)Ratio of partially occluded and total tubules. c)Significant difference where P≤0.05.
References
1. Banoczy J. Dentine hypersensitivity: general practice considerations for successful management. Int Dent J. 2002; 52:Suppl 5. 366.
2. Johnson RH, Zulqar-Nain BJ, Koval JJ. The effectiveness of an electro-ionizing toothbrush in the control of dentinal hypersensitivity. J Periodontol. 1982; 53:353–359.

3. Curro FA. Tooth hypersensitivity in the spectrum of pain. Dent Clin North Am. 1990; 34:429–437.
4. Bamise CT, Olusile AO, Oginni AO. An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract. 2008; 9:52–59.

5. Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity - an enigma? A review of terminology, mechanisms, aetiology and management. Br Dent J. 1999; 187:606–611.

6. Narhi MV. Responses of pulpal nociceptors to tissue injury and inflammation. In : Addy M, Embery G, Edgar WM, Orchardson R, editors. Tooth wear and sensitivity: clinical advances in restorative dentistry. London: Martin Dunitz;2000. p. 257–266.
7. Jacobsen PL, Bruce G. Clinical dentin hypersensitivity: understanding the causes and prescribing a treatment. J Contemp Dent Pract. 2001; 2:1–12.

8. Orchardson R. Strategies for the management of dentine hypersensitivity. In : Addy M, Embery G, Edgar WM, Orchardson R, editors. Tooth wear and sensitivity: clinical advances in restorative dentistry. London: Martin Dunitz;2000. p. 315–325.
9. Litkowski L. Pilot clinical and in vitro studies evaluating NovaMin in desensitizing dentifrices [abstract]. Dent Res. 1998; 77:199.
10. Absi EG, Addy M, Adams D. Dentine hypersensitivity: a study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987; 14:280–284.

11. Yoshiyama M, Masada J, Uchida A, Ishida H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res. 1989; 68:1498–1502.

13. West NX. Dentine hypersensitivity: preventive and therapeutic approaches to treatment. Periodontol 2000. 2008; 48:31–41.
14. Ling TY, Gillam DG, Barber PM, Mordan NJ, Critchell J. An investigation of potential desensitizing agents in the dentine disc model: a scanning electron microscopy study. J Oral Rehabil. 1997; 24:191–203.

15. Morris MF, Davis RD, Richardson BW. Clinical efficacy of two dentin desensitizing agents. Am J Dent. 1999; 12:72–76.
16. Gillam DG, Mordan NJ, Sinodinou AD, Tang JY, Knowles JC, Gibson IR. The effects of oxalate-containing products on the exposed dentine surface: an SEM investigation. J Oral Rehabil. 2001; 28:1037–1044.

17. Arrais CA, Chan DC, Giannini M. Effects of desensitizing agents on dentinal tubule occlusion. J Appl Oral Sci. 2004; 12:144–148.

18.
LJ Litkowski
GD Hack
DC Greenspan
. US Biomaterials Co.Compositions containing bioactive glass and their use in treating tooth hypersensitivity. United States patent. US 5,735,942. 1998. Apr. 07.
19.
LJ Litkowski
GD Hack
DC Greenspan
. US Biomaterials Co.Methods of treatment using bio-active glass. United States patent. US 6,086,374. 2000. Jul. 11.
21. Rajesh KS, Hedge S, Arun Kumar MS, Shetty DG. Evaluation of the efficacy of a 5% calcium sodium phosphosilicate (Novamin) containing dentifrice for the relief of dentinal hypersensitivity: a clinical study. Indian J Dent Res. 2012; 23:363–367.

22. Kolker JL, Vargas MA, Armstrong SR, Dawson DV. Effect of desensitizing agents on dentin permeability and dentin tubule occlusion. J Adhes Dent. 2002; 4:211–221.
23. Schüpbach P, Lutz F, Finger WJ. Closing of dentinal tubules by Gluma desensitizer. Eur J Oral Sci. 1997; 105(5 Pt 1):414–421.

24. In-Vitro evaluation of NovaMin root conditioner. Internal Research Report [Internet]. Alachua (FL): NovaMin Technology Inc.;cited 2013 Mar 15. Available from: http://www.oralscience.ca/en/documentation/articles/tooth_paste/In-Vitro-Evaluation-of-NovaMin-Root-Conditioner.pdf.
25. Du Min Q, Bian Z, Jiang H, Greenspan DC, Burwell AK, Zhong J, et al. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am J Dent. 2008; 21:210–214.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download