Abstract
A 47-year-old female previously diagnosed with ADPKD visited the hospital due to sudden pain in her upper abdomen and back. Esophagogastroduodenoscopy, contrast-enhanced abdominal computed tomography (CT), and CT angiography identified an esophageal artery pseudoaneurysm and hematoma in the esophagus. Urgent angiography and embolization were performed. After the procedure, CT angiography and positron emission tomography were performed due to differences in blood pressure between the arms. The patient was also found to have Takayasu arteritis and subsequently received outpatient follow-up care. The possible mechanisms that cause vascular abnormalities in ADPKD patients include damaged vascular integrity due to abnormal polycystin expression caused by PKD mutations and connective tissue abnormalities. Further research is needed to confirm these mechanisms, and ADPKD patients should be assessed for vascular abnormalities.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease and is characterized by numerous cysts in both kidneys1). In addition to affecting the kidneys, ADPKD is associated with various extrarenal vascular abnormalities including cerebral aneurysms, coronary artery aneurysms and dissection, cervicocephalic artery dissection, and aortic aneurysms and dissection23). Visceral artery aneurysms are rare; although gastroduodenal artery pseudoaneurysm and splenic artery aneurysm have been reported, no previous case of an esophageal artery pseudoaneurysm has been reported4).
Takayasu arteritis is a chronic granulomatous, large-vessel vasculitis primarily involving the aorta, its main branches, and the pulmonary arteries. Most arterial lesions in Takayasu arteritis are stenotic, whereas aneurysms can be found at various sites, including the visceral abdominal arteries56).
We present the case of an ADPKD patient who was diagnosed with an esophageal artery pseudoaneurysm and Takayasu arteritis.
A 47-year-old female visited the emergency room due to sudden pain in her upper abdomen and back. The patient had been diagnosed with ADPKD and severe polycystic liver disease 13 years prior (Fig. 1) and had received follow-up care with no symptoms. Additionally, as a complication of ADPKD, an arachnoid cyst in the left middle cranial fossa was found on brain magnetic resonance imaging.
The patient's blood pressure at the time of hospitalization was 140/100mm Hg, her heart rate was 62 beats per minute, her respiratory rate was 18 breaths per minute, and her temperature was 36.3℃. The laboratory findings showed a white blood cell count of 12,770/µL, a hemoglobin count of 13.5 g/dL, a platelet count of 184,000/µL, a blood urea nitrogen level of 15mg/dL, a creatinine level of 0.85 mg/dL, and a C-reactive protein level of 0.35mg/dL. Esophagogastroduodenoscopy showed external compression in the lower gastric body, but no hemorrhage of the gastroesophageal mucosa (Fig. 2).
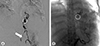
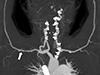
Computed tomography (CT) angiography showed an esophageal artery pseudoaneurysm and a hematoma in the esophagus (Fig. 3). Urgent angiography and embolization of the esophageal artery pseudoaneurysm were performed (Fig. 4). CT angiography was performed due to a blood pressure difference of 30mmHg (right arm, 124/77mmHg; left arm, 156/74mmHg) and the presence of a left subclavian bruit. This examination revealed that the patient also had Takayasu arteritis (Fig. 5). Autoantibody tests, including antineutrophilic cytoplasmic antibody, anticyclic citrullinated peptide antibody, and rheumatoid factor, were all negative. Positron emission tomography was performed to assess inflammation, but did not show any concerning signs of active vasculitis. The patient was transferred to outpatient management with low-dose aspirin (100mg once per day), and has not shown any signs of complications.
Both ADPKD and Takayasu arteritis can cause vascular complications, but involvement of the visceral arteries is uncommon47). There have been no reports of the simultaneous diagnosis of ADPKD and Takayasu arteritis, and this is also the first reported case of esophageal artery pseudoaneurysm in a patient with both ADPKD and Takayasu arteritis.
ADPKD is a hereditary disorder, and is accompanied by various systemic manifestations18). Among these, vascular abnormalities, such as cerebral or coronary aneurysm and aortic aneurysm and dissection, are relatively well-known, although the mechanisms are not entirely understood2). The possible mechanisms that cause vascular abnormalities in ADPKD patients include damaged vascular integrity due to abnormal polycystin expression caused by PKD mutations and connective tissue abnormalities.
ADPKD is known to be caused by mutations in the PKD1 and PKD2 genes, which code for the polycystin 1 and polycystin 2 proteins, respectively9). These mutations cause the growth of numerous renal and extrarenal cysts in ADPKD patients8). Studies have suggested that the vascular abnormalities found in ADPKD have to do with the polycystin 1 and polycystin 2 present in the vascular smooth muscles and endothelium and their involvement in the mechanism of vascular remodeling and early aneurysm development2310).
Researchers have long suspected that ADPKD is a type of connective tissue disorder. The histopathological findings of ADPKD patients are generally suggestive of connective tissue disorders. Based on vessel aneurysmal changes in the renal tissue, as well as excessive collagen matrix and edema seen in an ADPKD patient who underwent nephrectomy, it was suggested that weak, excessive collagen is the common pathogenic factor that is responsible for various ADPKD symptoms11). Moreover, in an autopsy of an individual with vertebrobasilar dolichoectasia, changes were observed in the integrity of the extracellular matrix, with degeneration and multiple gaps in the internal elastic lamina, thinning of the media, and smooth muscle atrophy; these observations were considered to provide grounds for seeing ADPKD as a connective tissue disorder12).
Takayasu arteritis is a systemic inflammatory vasculitis of unknown etiology. The pathogenesis of Takayasu arteritis is poorly understood, but T cell-mediated mechanisms are thought to be the most important13). The inflammatory process within the vessel can result in stenosis, occlusion, dilatation, or aneurysm formation in the involved portions of the arteries5). Although many studies have investigated Takayasu arteritis, its diagnosis is still challenging. This patient fulfilled 3 of 6 American College of Rheumatology criteria for the diagnosis of Takayasu arteritis14): difference in blood pressure between arms, the presence of a left subclavian bruit, and arteriographic abnormalities. However, to meet the modified Ishikawa diagnostic criteria, esophageal artery pseudoaneurysm should be included5).
The differential diagnosis of esophageal artery pseudoaneurysm includes atherosclerosis, connective tissue disorders (e.g., Marfan, Ehlers-Danlos, or fibromuscular dysplasia), vasculitis (e.g., polyarteritis nodosa or Takayasu arteritis), and polycystic kidney disease15). In this case, the esophageal artery pseudoaneurysm may have been caused by either ADPKD or Takayasu arteritis. We reviewed the patient's images before the onset of symptoms and found celiac axis stenosis, which can be a manifestation of Takayasu arteritis.
Since this was the first report of ADPKD and Takayasu arteritis in a single patient, it was not easy to identify a possible link between the 2 diseases. Baek et al.16) proposed the possibility of a T cell-mediated autoimmune response to an abnormal extracellular matrix protein resulting from the mutation implicated in Marfan syndrome, with regard to the pathogenesis of a case of Marfan syndrome accompanied by Takayasu arteritis. In this report, we suggest that Takayasu arteritis may be caused by a T cell-mediated autoimmune response to abnormal polycystin proteins, similar to the proposal by Baek et al.
We presented a novel and interesting case of esophageal artery pseudoaneurysm in a patient with ADPKD, proving that ADPKD is a type of connective tissue disorder. Vascular abnormalities are a major complication of ADPKD, but if rare vascular complications occur in ADPKD patients, it is important to reassess the possibility of other forms of vasculitis or another connective tissue disorder. Further study is required to confirm the relevant mechanisms.
Figures and Tables
Fig. 1
Contrast-enhanced abdominopelvic computed tomography, showing numerous cysts in the liver and both kidneys.
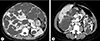
Fig. 2
Esophagogastroduodenoscopy. External compression is noted at the lower body, antrum, and duodenal bulb (arrowheads).
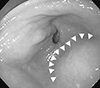
Fig. 3
Computed tomography angiography, showing multifocal pseudoaneurysms (arrowheads) of the esophageal artery from the left gastric artery (arrow), with a suspected hematoma along the esophageal wall.
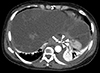
Fig. 4
Angiography (arteriography of the superior mesenteric artery). A 9-mm pseudoaneurysm(circle) was found in the esophageal artery and it was confirmed that it communicated with the distal left gastric artery (arrow). The distal portion of the esophageal artery was embolized and the esophageal artery was no longer seen (dashed circle) on angiography after the procedure.
Acknowledgments
This research was supported by a grant from the National Research Foundation, Republic of Korea (grant number: NRF-2016R1D1A1B03934173).
References
2. Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015; 11:589–598.

3. Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009; 5:221–228.

4. Uğur M, Ersoy E, Oztepe B, Kinay E, Sezer S. Rare Etiology of Gastrointestinal Bleeding: Polycystic Kidney Disease. Gavin J Urol Renal Dis. 2016; 2016:13–15.
5. de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun. 2014; 48:79–83.

6. Matsumura K, Hirano T, Takeda K, Matsuda A, Nakagawa T, Yamaguchi N, et al. Incidence of aneurysms in Takayasu's arteritis. Angiology. 1991; 42:308–315.

7. Matsumoto T, Ishizuka M, Iso Y, Kita J, Kubota K. Mini-Laparotomy for Superior Mesenteric Artery Aneurysm Due to Takayasu's Arteritis. Int Surg. 2015; 100:765–769.

8. Sung PH, Yang YH, Chiang HJ, Chiang JY, Chen CJ, Liu CT, et al. Risk of aortic aneurysm and dissection in patients with autosomal-dominant polycystic kidney disease: a nationwide population-based cohort study. Oncotarget. 2017; 8:57594.

9. Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002; 13:2384–2398.

10. Graf S, Schischma A, Eberhardt KE, Istel R, Stiasny B, Schulze BD. Intracranial aneurysms and dolichoectasia in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2002; 17:819–823.

11. Ul Haque A. Adult polycystic kidney disease: a disorder of connective tissue? Int J Clin Exp Pathol. 2008; 1:84.
12. Nakagawa S, Furuichi K, Sagara A, Shinozaki Y, Kitajima S, Toyama T, et al. An autopsy case of vertebrobasilar dolichoectasia under hemodialysis due to autosomal dominant polycystic kidney disease. CEN Case Rep. 2016; 5:51–55.

13. Serra R, Butrico L, Fugetto F, Chibireva MD, Malva A, De Caridi G, et al. Updates in Pathophysiology, Diagnosis and Management of Takayasu Arteritis. Ann Vasc Surg. 2016; 35:210–225.

14. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 33:1129–1134.

15. van Rijn MJ, ten Raa S, Hendriks J, Verhagen H. Visceral aneurysms: Old paradigms, new insights? Best Pract Res Clin Gastroenterol. 2017; 31:97–104.

16. Baek HJ, Shin KC, Lee YJ, Kang SW, Lee EB, Song YW. Takayasu's arteritis concurrent with Marfan syndrome--a case report. Angiology. 2000; 51:435–439.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download