Abstract
Proteinuria is a major promoter that induces tubulointerstitial injury in glomerulopathy. Dietary salt restriction may reduce proteinuria, although the mechanism is not clear. We investigated the effects of dietary salt restriction on rat kidneys in an animal model of glomerular proteinuria. Male Sprague-Dawley rats were used and divided into 3 groups: vehicle-treated normal-salt controls, puromycin aminonucleoside (PA)-treated normal-salt rats, and PA-treated low-salt rats. PA was given at a dose of 150 mg/kg BW at time 0, followed by 50 mg/kg BW on days 28, 35, and 42. Sodium-deficient rodent diet with and without additional NaCl (0.5%) were provided for normal-salt rats and low-salt rats, respectively. On day 63, kidneys were harvested for histopathologic examination and immunohistochemistry. PA treatment produced overt proteinuria and renal damage. Dietary salt restriction insignificantly reduced proteinuria in PA-treated rats, and PA-treated low-salt rats had lower urine output and lower creatinine clearance than vehicle-treated normal-salt controls. When tubulointerstitial injury was semiquantitatively evaluated, it had a positive correlation with proteinuria. The tubulointerstitial injury score was significantly increased by PA treatment and relieved by low-salt diet. ED1-positive infiltrating cells and immunostaining for interstitial collagen III were significantly increased by PA treatment. These changes appeared to be less common in PA-treated low-salt rats, although the differences in PA-treated normal-salt versus low-salt rats did not reach statistical significance. Our results suggest that renal histopathology in PA nephrosis may potentially be improved by dietary salt restriction. Non-hemodynamic mechanisms induced by low-sodium diet might contribute to renoprotection.
In glomerular disease, variable degrees of tubulointerstitial injury and fibrosis coexist. The tubulointerstitial fibrosis is prominent in glomerular diseases that progress to chronic renal failure, and the glomerular filtration rate correlates better with tubulointerstitial injury than with glomerular injury1-3).
Glomerular proteinuria is a risk factor for both progression of chronic renal failure and interstitial fibrosis, suggesting a causative relationship between glomerular protein ultrafiltration and interstitial fibrogenesis. Thus, proteinuria or albuminuria is not only just a marker for the glomerular ultrafiltration, but also an important mediator for tubular actions of ultrafiltered, biologically active growth factors which stimulate tubular cells causing basolateral secretion of chemokines and cytokines. Chemokines attract and activate macrophages. Tubular cell-derived platelet-derived growth factor (PDGF) and macrophage-derived transforming growth factor-β (TGF-β) cause fibroblast proliferation. Several growth factors contribute to their transition into extracellular matrix-producing myofibroblasts4).
High sodium intake increases blood pressure and proteinuria, induces glomerular hyperfiltration and blunts the response to renin-angiotensin-aldosterone system (RAAS) blockade5). Besides, dietary salt intake may have a role in modulating intrarenal production of TGF-β6-8). The mechanism is independent of angiotensin II and systemic blood pressure and involves activation of vascular endothelium by dietary salt intake with release of this growth factor9). It is now clear that TGF-β has a potent role in chronic progression of renal disease10), and that Smad3 is critical for TGF-β's pro-fibrotic effect11).
On the other hand, the effects of low sodium intake on renal progression are not clear. It is conceivable that strict restriction of dietary salt intake activates RAAS, precipitating renal progression. This study was undertaken to test if dietary salt restriction may modulate glomerular proteinuria-induced tubulointerstitial damage using a rat model of puromycin aminonucleoside nephrosis.
Specific pathogen-free male Sprague-Dawley rats (Orient Bio Inc., Seongnam, Korea) weighing 120-150 g, were used for induction of puromycin aminonuceloside nephrosis. Rats were divided into 3 groups: vehicle-treated normal-salt controls (n = 3), puromycin aminonucleoside (PA)-treated normal-salt rats (n = 3), and PA-treated low-salt rats (n = 3). PA (Sigma, St. Louis, MO, USA) was dissolved in 0.9% saline (vehicle) and used at a concentration of 10 mg/mL.
On day 0, PA-treated rats were given an intraperitoneal injection of PA 150 mg/kg BW, and vehicle-treated normal-salt controls were given an intraperitoneal injection of the same volume of 0.9% saline. Second, third and fourth doses of PAN (50 mg/kg BW i.p.) were administered to the PA-treated rats at 4, 5 and 6 weeks after the initial dose; the control rats received i.p. saline at the same times.
All rats were maintained on a food slurry, a mixture of base diet (18 g/200 g BW) and deionized water (9 mL/200 g BW). The base diet was a commercially available synthetic rat chow containing no added NaCl (DYET #113763 Sodium Deficient AIN-93G Purified Rodent Diet, Dyets Inc., Bethlehem, PA, USA) and offered for low-salt rats. Normal-salt rats were fed the base diet containing added NaCl (0.5%). Furthermore, drinking water was freely accessible to all rats.
Animals were housed individually in metabolic cages to provide a daily fixed amount of food and to obtain 24-hour collections of urine. The animal experiment was terminated on day 63, and kidneys were harvested for histopathologic examination and immunohistochemistry. The experimental protocol was approved by the institutional Animal Care and Use Committee of Hanyang University.
Renal tissues were fixed in 10% neutral-buffered formalin and were embedded in paraffin. Four-micron paraffin sections were stained with hematoxylin and periodic acid-Schiff (PAS) or with Masson's trichrome. Tubulointerstitial injury was scored semiquantitatively on PAS-stained tissues, with a 20X objective, as described previously12). Tubulointerstitial injury was defined as tubular dilation, tubular atrophy, sloughing of tubular epithelial cells, or thickening of the tubular basement membrane and was scored on a scale of 0 to 4, as follows: 0, no tubulointerstitial injury; 1, < 25% of the tubulointerstitium injured; 2, 25 to 50% of the tubulointerstitium injured; 3, 51 to 75% of the tubulointerstitium injured; 4, > 75% of the tubulointerstitium injured.
The extent of tubulointerstitial scarring was evaluated on trichrome-stained sections to assess the relative proportion of interstitial fibrosis. For each animal, 15 photographs of random areas of cortex and medulla are taken at 100X magnification; the photographs are converted to JPEG files using a scanner, at a resolution of 200 pixels/inch. The relative fraction of red and blue pixels in each image was quantified using SigmaScan software13).
The kidneys were preserved by retrograde perfusion via the abdominal aorta with 1% phosphate-buffered saline (PBS) to remove blood and then with periodate-lysine-paraformaldehyde (PLP; 0.01 M NaIO4, 0.075 M lysine, 2% paraformaldehyde, in 0.0375 M Na2HPO4 buffer, pH 6.2) for kidney fixation for 3 minutes. After completion of perfusion, each kidney was sliced into 5 mm thick sections and immersed in 2% PLP solution overnight at 4℃.
Immunohistochemical staining for osteopontin (OPN) protein and macrophage accumulation was performed on formalin-fixed, paraffin-embedded sections using a microwave antigen retrieval method14). Sections were deparaffined with a graded series of ethanol, and treated with 14 minutes of microwave oven heating in 0.01 mM sodium citrate, pH 6.0, at 2,450 MHz and 800W. Endogenous peroxidase was inactivated by incubation in 3% H2O2 in methanol. After preincubation with 10% normal goat serum (S-1000; Vector Laboratory, Burlington, CA) for 1 hour, sections were incubated overnight at 4℃ with mouse mAbs to rat OPN (MPIIIB10; obtained from the Developmental Studies Hybridoma Bank, Iowa City, IA, USA) or rat macrophages (ED1, Serotec, Oxford, UK). For ED1 IHC, pretreatment with trypsin (Sigma) was done at 37℃ for 15 minutes. They were washed with PBS and incubated for 2 hours with a DAKO Envision kit (DAKO, Glostrup, Denmark) at room temperature. Sections then were washed in 0.05M Tris buffer, and developed with a mixture of 3,3'-diaminobenzidine and 0.033% H2O2 in Tris buffer. They were were counter-stained with hematoxylin, and slides were mounted with Canadian balasam (Sigma, St. Louis, MO, USA).
OPN protein expression was assessed quantitatively by measuring the area of immunostaining for OPN using a computerized image analyzer (Leica Application Suite; Leica Microsystems, Wetzlar, Germany). This was performed by assessing all fields under a 20X objective encountered in tracing a serpentine course in a section of kidney15). A point-counting technique was used to quantitate the number of interstitial ED1-positive cells. Interstitial ED1-positive macrophages were counted in at least 20 consecutive high-power fields14).
At the end of the animal experiment, there were no differences in body weight between the groups: vehicle-treated normal-salt controls, 332 ± 1 g; PA-treated normal-salt rats, 297 ± 13 g; and PA-treated low-salt rats, 312 ± 7 g. The 3 groups also had no differences in weight gain over the study period.
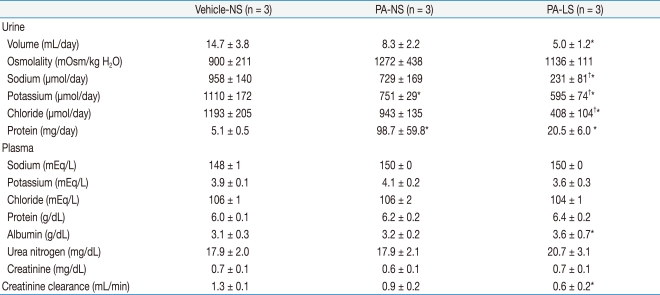
Urine and plasma data obtained on the final day of the animal experiment are shown in Table 1. Urine volume was not significantly affected by PA administration, but PA-treated low-salt rats had smaller urine output than vehicle-treated normal-salt controls (5.0 ± 1.2 versus 14.7 ± 3.8 mL/day, P < 0.05). However, there were no differences in urine osmolality between the groups. As expected, PA-treated low-salt rats had smaller urinary excretions of sodium and chloride. Interestingly, urinary potassium excretion was significantly decreased by PA administration, irrespective of dietary salt restriction. However, plasma sodium, potassium and chloride concentrations showed no differences between the groups.
Compared with vehicle-treated normal-salt controls, proteinuria was increased in PA-treated normal-salt rats (5.1 ± 0.5 versus 98.7 ± 59.8 mg/day, P < 0.05). Although the mean value of proteinuria in PA-treated low-salt rats (20.5 ± 6.0 mg/day) was lower than in PA-treated normal-salt rats, the difference did not reach statistical significance. Interestingly, the plasma albumin level was significantly increased in PA-treated low-salt rats compared with vehicle-treated normal-salt controls (3.6 ± 0.7 versus 3.1 ± 0.3 g/dL, P < 0.05). Although blood urea nitrogen and plasma creatinine were not different between the groups, creatinine clearance was significantly lowered in PA-treated low-salt rats compared with vehicle-treated normal-salt controls (0.6 ± 0.2 versus 1.3 ± 0.1 mL/min, P < 0.05).
Nine weeks after 4 times' PA administration, overt tubulointerstitial abnormalities were noted in the kidney. They included interstitial edema and inflammatory cell infiltration, tubular atrophy and dilatation, and intratubular casts with intraluminal cellular debris. When these were semiquantitatively evaluated, the results were: vehicle-treated normal-salt controls, 0.2 ± 0.0; PA-treated normal-salt rats, 3.2 ± 0.4; and PA-treated low-salt rats, 1.7 ± 0.2. There were significant differences in tubulointerstitial injury score between the groups, and PA-treated low-salt rat kidneys showed significantly (P < 0.05) less tubulointerstitial injury than PA-treated normal-salt rats (Fig. 1). In contrast, glomeruli showed only minor changes such as mild hypercellularity in PA-treated rat kidneys.
The relationship between proteinuria and tubulointerstitial injury was demonstrated in Figure 2. The degree of proteinuria correlates positively with that of tubulointerstitial injury (r=0.76, P < 0.05).
Figure 3 shows ED1 immunohistochemistry illustrative of macrophage infiltration. PA treatment induced a remarkable increase in the number of ED1-positive cells in the kidney (345 ± 136 versus 2,106 ± 415/HPF, P < 0.05). With dietary salt restriction, the increased macrophage infiltration was insignificantly reversed (1,214 ± 268/HPF).
Figure 4 shows OPN immunohistochemistry from vehicle-treated normal-salt controls, PA-treated normal-salt rats, and PA-treated low-salt rats. When the area of immunostaining for OPN was quantitatively assessed, the results were: vehicle-treated normal-salt controls, 0.12 ± 0.11 %area; PA-treated normal-salt rats, 0.51 ± 0.32%area; and PA-treated low-salt rats, 0.13 ± 0.07%area. Thus the renal expression of OPN seemed to be increased by PA treatment and relieved by low-salt diet. However, these changes were not statistically significant.
The interstitial accumulation of collagen III was significantly increased in PA-treated normal-salt rat kidneys compared with vehicle-treated normal-salt controls (3.12 ± 0.89 versus 0.81 ± 0.26 %area, P < 0.05). As compared with PA-treated normal-salt rats, PA-treated low-salt rat kidneys showed a decreasing tendency in interstitial collagen III immunostaining (1.67 ± 0.57 %area, Fig. 5) However, the difference was not statistically significant. When we examined blue-to-red pixel ratios on Masson's trichrome staining (Fig. 6), the results were: vehicle-treated normal-salt controls, 0.187 ± 0.053; PA-treated normal-salt rats, 0.252 ± 0.004; and PA-treated low-salt rats, 0.183 ± 0.053. There were no significant differences between the groups.
This preliminary study was designed to test whether low-sodium diet is beneficial to glomerular proteinuria-induced tubulointerstitial injury. To produce nephrotic syndrome, we used the animal model of chronic purine aminonucleoside nephrosis (PAN)16). With dietary salt restriction, PAN rats had insignificant reduction of proteinuria but showed significant improvement in tubulointerstitial injury in their kidneys.
Restriction of dietary sodium may reduce proteinuria probably via renal hemodynamic alteration5). In white salt-sensitive hypertensive patients, high sodium intake (250 mmol/day) was associated with an almost 35% increase in albuminuria as compared with low sodium intake (20 mmol/day)17). The antiproteinuric effect in black (mostly salt sensitive) hypertensive patients was 19% when re ducing dietary sodium intake18). In IgA nephropathy, reduction of dietary sodium from 12 to 5 g/day reduced proteinuria by approximately 30-50%19), and in patients with nondiabetic proteinuria, dietary sodium restriction (urinary sodium excretion from 200 to 90 mmol/24 hr) reduced proteinuria from 3.9 to 2.7 g/24 hr20). In these studies on hypertensive kidney disease, the reduction in proteinuria during low sodium intake was generally but not invariably associated with a lower blood pressure. In healthy individuals, on the contrary, reduction of dietary sodium from 200 to 50 mmol/24 hr significantly reduced urinary albumin excretion within the normal range, independent of BP, indicating specific renoprotective effects of dietary sodium restriction21-23). This is in line with the BP-independent association between urinary sodium excretion and albuminuria in the general population24). Regarding animal data, we could find no reports on dietary salt restriction in PAN rats.
We investigated non-hemodynamic renal mechanisms by which dietary salt restriction exerts renoprotection. The association between proteinuria and progression of chronic kidney disease is firmly established, and in this study we showed a correlation between proteinuria and tubulointerstitial injury. Tubular cells exposed to high concentration of proteins produce proinflammatory and profibrotic factors25). As biomarkers for this connection between glomerular proteinuria and tubulointerstitial inflammation, we examined the inflammatory cell infiltra tion and OPN expression in the tubulointerstitium. Pre viously, increased expression of OPN associated with in terstitial monocyte infiltration has been demonstrated in chronic PAN rat kidneys15). We also showed increased tubular OPN expression and interstitial ED1-positive cell infiltration in PAN rats. These changes appeared less in PA-treated low-salt rats although the differences in PA-treated normal-salt versus low-salt rats did not reach statistical significance.
TGF-β is the most important molecule to activate the fibrogenic cascades in the process of tubulointerstitial injury. High sodium intake increases renal expression of TGF-β6,26). However, the effect of low sodium diet compared with normal sodium intake is not clear. We tested if the TGF-β/Smad3 signaling pathway was affected in our animals, but no significant changes were found (data not shown).
We examined interstitial fibrosis by collagen III immunohistochemistry and Masson's trichrome staining. As expected, the interstitial accumulation of collagen III was significantly increased by PA treatment. This increase in collagen III expression seemed to be relieved by dietary salt restriction although the difference was not statistically significant. We believe that we obtained insignificant changes in the number of ED1-positive cells and immunostaining for OPN and collagen III between the groups because of the small number of animals used in this study. Further studies are required to resolve these issues.
On the other hand, we are concerned if too little salt intake might have negative consequences. Radical salt restriction causes hypovolemia, with the attendant adverse effects27). In this study, urine output and creatinine clearance tended to be lower in both PA-treated normal-salt rats and PA-treated low-salt rats. However, the change from vehicle-treated normal-salt controls was larger in PA-treated low-salt rats, and the differences in PA-treated low-salt rats only reached statistical significance. Thus, it is not clear whether the long-term renal outcome would be improved by low salt intake in glomerulopathy. Whereas dietary salt restriction may relieve glomerular hyperfiltration, it may up-regulate the activity of the renin-angiotensin system, increases angiotensin II, and up-regulate NADPH oxidase, thus, generating reactive oxidant species28). In both humans and animals, the optimal dietary salt deprivation needs to be elucidated for benefits versus risks.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00269).
References
1. Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987; 7:421–433. PMID: 3439552.

2. Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968; 2:363–366. PMID: 4173786.

3. Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970; 1:631–641. PMID: 5521736.
4. Hirschberg R, Wang S. Proteinuria and growth factors in the development of tubulointerstitial injury and scarring in kidney disease. Curr Opin Nephrol Hypertens. 2005; 14:43–52. PMID: 15586015.

5. Krikken JA, Laverman GD, Navis G. Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2009; 18:531–538. PMID: 19713840.

6. Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol. 1998; 274:F635–F641. PMID: 9575885.
7. Ying WZ, Sanders PW. Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-beta1. Am J Physiol. 1998; 275:F18–F24. PMID: 9689000.
8. Ying WZ, Sanders PW. Dietary salt intake activates MAP kinases in the rat kidney. FASEB J. 2002; 16:1683–1684. PMID: 12206991.
9. Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008; 295:F406–F414. PMID: 18562633.
10. Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl. 2000; 77:S45–S52. PMID: 10997690.
11. Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton). 2005; 10:48–56. PMID: 15705182.
12. Nangaku M, Pippin J, Couser WG. Complement membrane attack complex (C5b-9) mediates interstitial disease in experimental nephrotic syndrome. J Am Soc Nephrol. 1999; 10:2323–2331. PMID: 10541291.

13. Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D, Liang LF. Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma. 2010; 69:579–583. PMID: 20173658.

14. Yu XQ, Wu LL, Huang XR, et al. Osteopontin expression in progressive renal injury in remnant kidney: role of angiotensin II. Kidney Int. 2000; 58:1469–1480. PMID: 11012882.

15. Magil AB, Pichler RH, Johnson RJ. Osteopontin in chronic puromycin aminonucleoside nephrosis. J Am Soc Nephrol. 1997; 8:1383–1390. PMID: 9294829.

16. Jones CL, Buch S, Post M, McCulloch L, Liu E, Eddy AA. Pathogenesis of interstitial fibrosis in chronic purine aminonucleoside nephrosis. Kidney Int. 1991; 40:1020–1031. PMID: 1762303.

17. Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994; 23:195–199. PMID: 8307628.

18. Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005; 46:308–312. PMID: 15983240.
19. Konishi Y, Okada N, Okamura M, et al. Sodium sensitivity of blood pressure appearing before hypertension and related to histological damage in immunoglobulin a nephropathy. Hypertension. 2001; 38:81–85. PMID: 11463764.

20. Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008; 19:999–1007. PMID: 18272844.

21. Krikken JA, Lely AT, Bakker SJ, Navis G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int. 2007; 71:260–265. PMID: 17091123.

22. Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D. Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int. 1989; 36:272–279. PMID: 2550696.

23. Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998; 13:1682–1685. PMID: 9681711.

24. Verhave JC, Hillege HL, Burgerhof JG, et al. Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004; 256:324–330. PMID: 15367175.

25. Rodriguez-Iturbe B, Garcia Garcia G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract. 2010; 116:c81–c88. PMID: 20502043.

26. Tamaki K, Okuda S, Nakayama M, Yanagida T, Fujishima M. Transforming growth factor-beta 1 in hypertensive renal injury in Dahl salt-sensitive rats. J Am Soc Nephrol. 1996; 7:2578–2589. PMID: 8989736.

27. Ritz E, Mehls O. Salt restriction in kidney disease--a missed therapeutic opportunity? Pediatr Nephrol. 2009; 24:9–17. PMID: 18535843.

28. Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002; 346:1999–2001. PMID: 12075063.

Fig. 1
Histopathology of the Kidney Using Periodic AcidSchiff (PAS) Staining. Representative sections are shown from each group (A), and results of semiquantitative evaluation of tubulointerstitial injury are summarized in a bar graph (B). Magnification ×200. PA, puromycin aminonucleoside; NS, normalsalt diet; LS, lowsalt diet.
*P < 0.05 versus VehicleNS.
†P < 0.05 versus PANS.
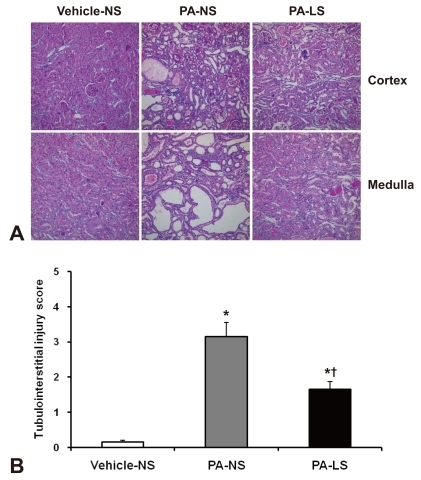
Fig. 2
Correlation between Proteinuria and Tubulointerstitial Injury Score. Tubulointerstitial injury correlates positively with proteinuria in this animal experiment.
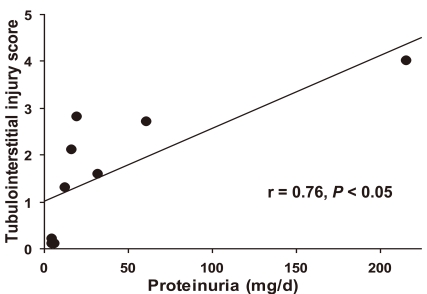
Fig. 3
Immunohistochemistry for ED1 in Rat Kidneys. Representative sections are shown from each group (A), and results of quantitative evaluation of immunostaining are summarized in a bar graph (B). Magnification ×200. PA, puromycin aminonucleoside; NS, normalsalt diet; LS, lowsalt diet.
*P < 0.05 versus VehicleNS.
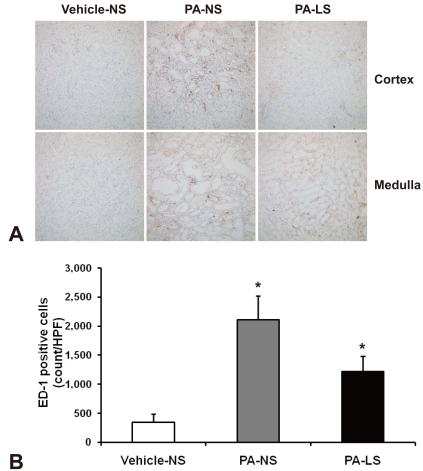
Fig. 4
Immunohistochemistry for Osteopontin in Rat Kidneys. Representative sections are shown from each group (A), and results of quantitative evaluation of immunostaining are summarized in a bar graph (B). No significant differences were noted between groups. Magnification ×200. PA, puromycin aminonucleoside; NS, normalsalt diet; LS, lowsalt diet.
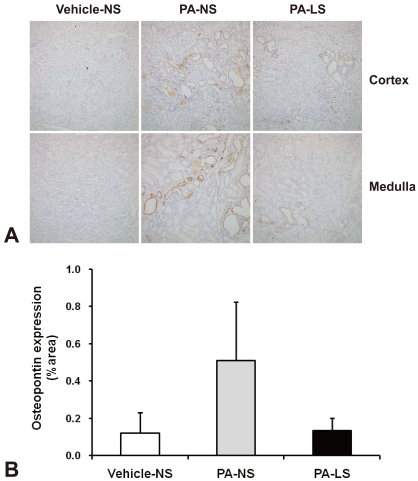
Fig. 5
Immunohistochemistry for Collagen III in Rat Kidneys. Representative sections are shown from each group (A), and results of quantitative evaluation of immunostaining are summarized in a bar graph (B). Magnification ×200. PA, puromycin aminonucleoside; NS, normalsalt diet; LS, lowsalt diet.
*P < 0.05 versus VehicleNS.
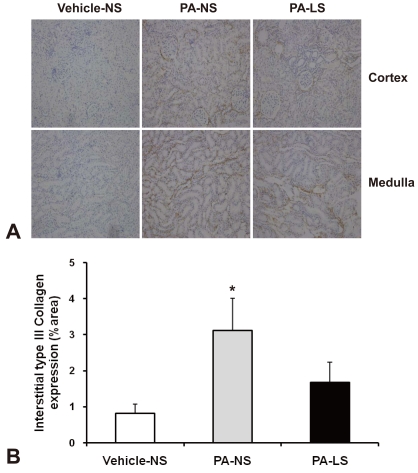
Fig. 6
Histopathology of the Kidney Using Masson's Trichrome Staining. Representative sections are shown from each group (A), and bluetored pixel ratios are expressed in a bar graph (B). No significant differences were noted between groups. Magnification ×200. PA, puromycin aminonucleoside; NS, normalsalt diet; LS, lowsalt diet.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download