Abstract
Background:
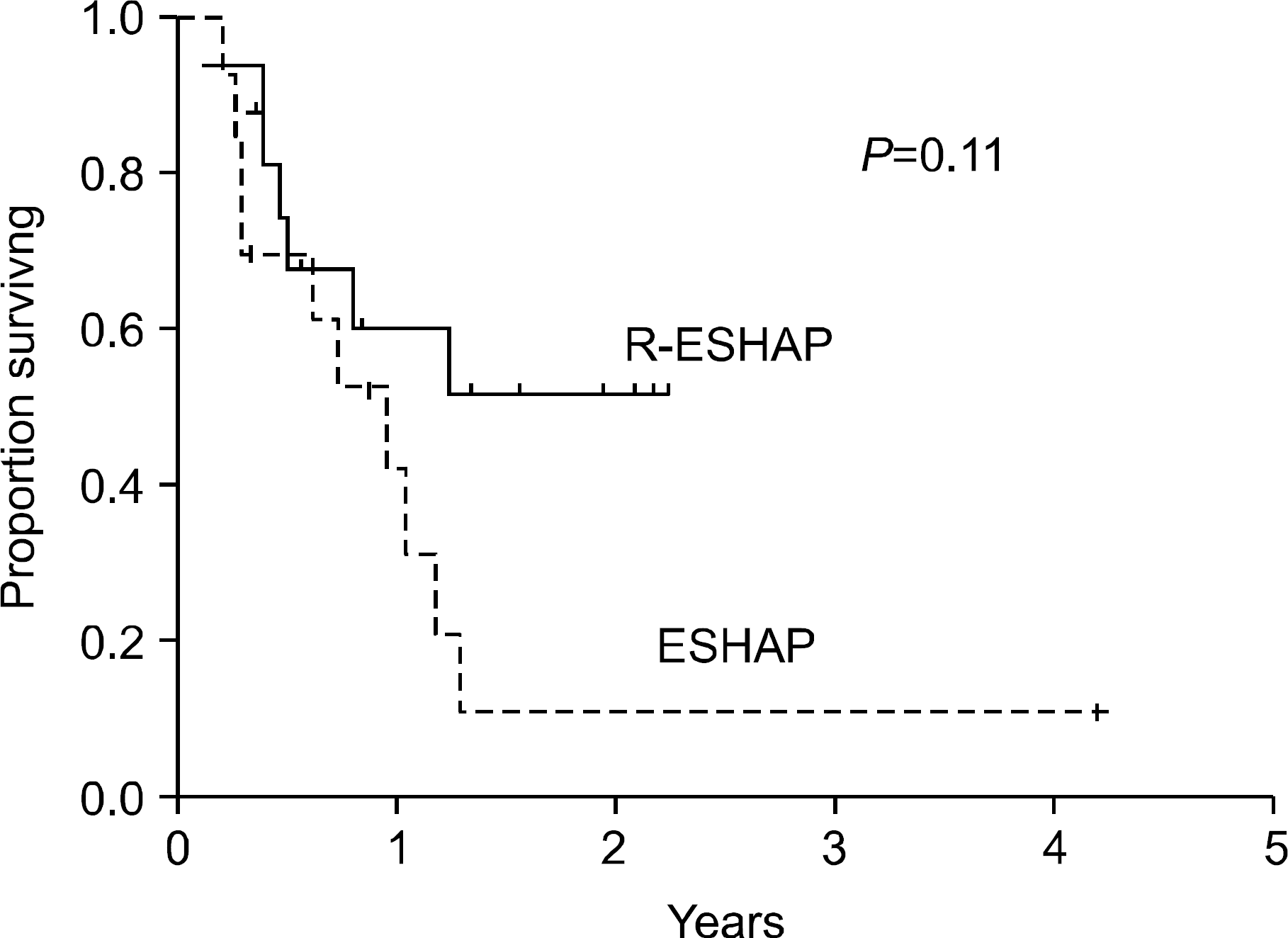
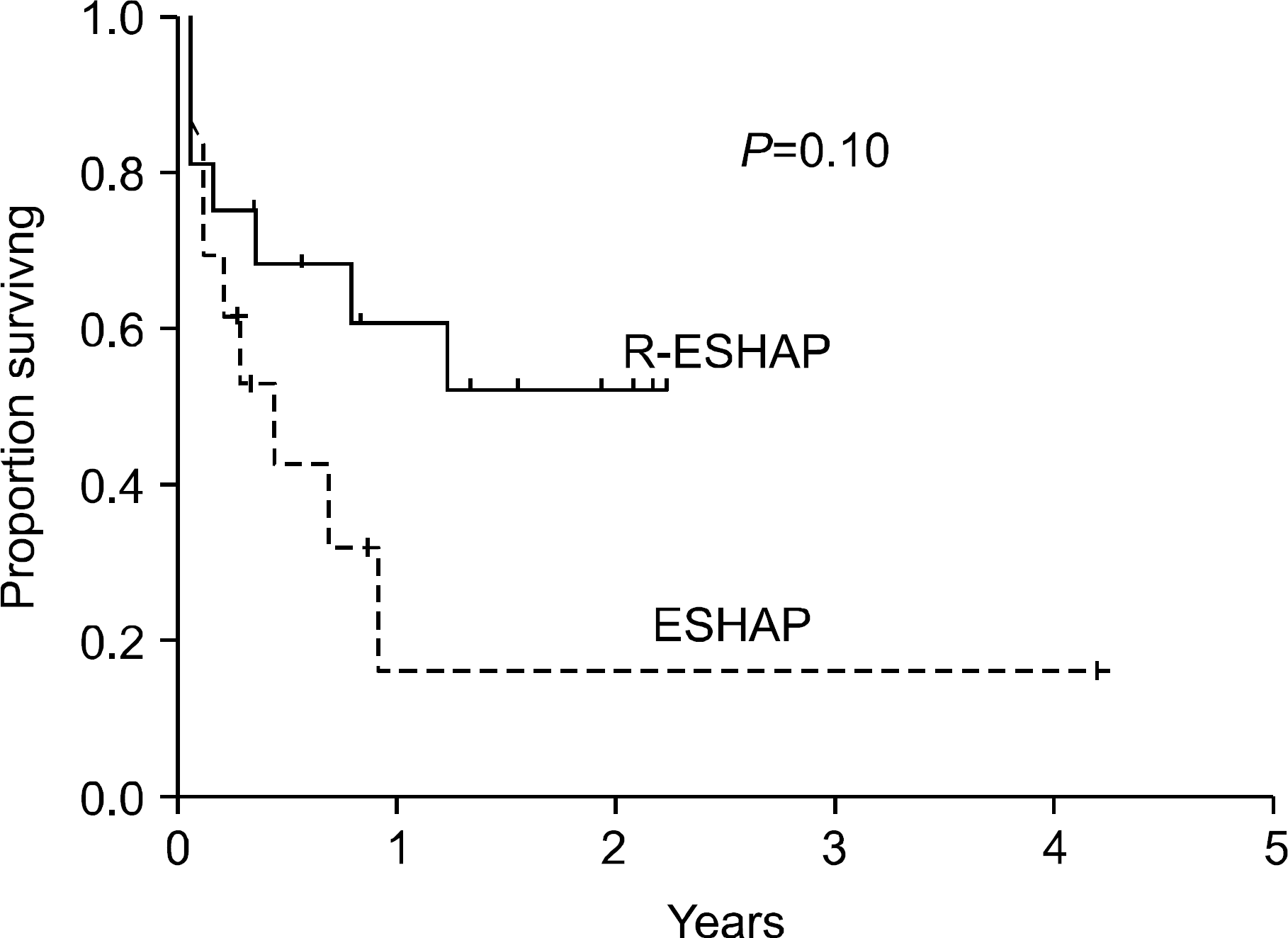
The remission status prior to autologous stem cell transplantation (ASCT) influences the transplantation outcome in patients with relapsed or primary refractory diffuse large B cell lymphoma (DLBCL), a complete response (CR) generally being more favorable than a partial response (PR). This study investigated whether the addition of rituximab to the ESHAP chemotherapy regimen (R-ESHAP) could improve the CR rate in patients with relapsed or primary refractory DLBCL.
Results:
Sixteen patients who had previously received one course of chemotherapy were administered R-ESHAP (median 3 cycles; range 1∼6). The overall response rate of 75% (CR=50%; PR=25%), was significantly better than that achieved with ESHAP alone in 13 historical controls (31%; P=0.027). The toxicity was tolerable, with two febrile neutropenia episodes in 51 treatment cycles. Seven of the 12 responders to R-ESHAP underwent ASCT with BEAM. After a median follow-up of 17 months, the median survival endpoints have not been reached.
REFERENCES
1). Fisher RI., Gaynor ER., Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993. 328:1002–6.

2). Gordon LI., Harrington D., Andersen J, et al. Comparison of a second-generation combination chemo-the-rapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin's lymphoma. N Engl J Med. 1992. 327:1342–9.

3). Coiffier B., Lepage E., Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:235–42.

4). Velasquez WS., Cabanillas F., Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988. 71:117–22.

5). Velasquez WS., McLaughlin P., Tucker S, et al. ESHAP ? an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994. 12:1169–76.
6). Gisselbrecht C., Mounier N. Improving second-line therapy in aggressive non-Hodgkin's lymphoma. Semin Oncol. 2004. 31(2 Suppl):12–6.

7). Coiffier B., Haioun C., Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998. 92:1927–32.
8). Kewalramani T., Zelenetz AD., Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004. 103:3684–8.

9). Philip T., Guglielmi C., Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995. 333:1540–5.

10). Prince HM., Imrie K., Crump M, et al. The role of intensive therapy and autologous blood and marrow transplantation for chemotherapy-sensitive relapsed and primary refractory non-Hodgkin's lymphoma: identification of major prognostic groups. Br J Haematol. 1996. 92:880–9.

11). Choi CW., Paek CW., Seo JH, et al. ESHAP salvage therapy for relapsed or refractory non-Hodgkin's lymphoma. J Korean Med Sci. 2002. 17:621–4.

12). Lee JL., Kim S., Kim SW, et al. ESHAP plus G-CSF as an effective peripheral blood progenitor cell mobilization regimen in pretreated non-Hodgkin's lymphoma: comparison with high-dose cyclophosphamide plus G-CSF. Bone Marrow Transplant. 2005. 35:449–54.

13). Harris NL., Jaffe ES., Diebold J, et al. World Health Organization Classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting ? Airlie House, Virginia, November 1997. J Clin Oncol. 1999. 17:3835–49.
14). Cheson BD., Horning SJ., Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999. 17:1244.
15). Kim S., Kim HJ., Park JS, et al. Prospective randomized comparative observation of single vs split-dose lenograstim to mobilize peripheral blood progenitor cells following chemotherapy in patients with multiple myeloma or non-Hodgkin's lymphoma. Ann Hematol. 2005. 84:742–7.

16). Suh C., Kim S., Kim SH, et al. Initiation of peripheral blood progenitor cell harvest based on peripheral blood hematopoietic progenitor cell counts enumerated by the Sysmex SE9000. Transfusion. 2004. 44:1762–8.

17). Caballero MD., Rubio V., Rifon J, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997. 20:451–8.

18). Caballero MD., Perez-Simon JA., Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003. 14:140–51.

19). Moskowitz CH., Bertino JR., Glassman JR, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. 1999. 17:3776–85.
20). Vose JM., Zhang MJ., Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001. 19:406–13.

21). Demidem A., Lam T., Alas S., Hariharan K., Hanna N., Bonavida B. Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm. 1997. 12:177–86.

22). Alas S., Ng CP., Bonavida B. Rituximab modifies the cisplatin?mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkin's lymphoma. Clin Cancer Res. 2002. 8:836–45.
23). Emmanouilides C., Jazirehi AR., Bonavida B. Rituximab-mediated sensitization of B-non-Hodgkin's lymphoma (NHL) to cytotoxicity induced by paclitaxel, gemcitabine, and vinorelbine. Cancer Biother Radiopharm. 2002. 17:621–30.

24). Arnold C., Cuthbert R., Morris TCM., Kettle P., Jones F., Drake M. Rituximab-ESHAP as salvage therapy in relapsed aggressive B cell lymphoma [abstract]. Haematologica. 2005. 90(2 Suppl):): Abstract. 1146.
25). Piliotis E., Mangel J., Buckstein R., Imrie K., Spaner D., Reis M. A Phase II trial of rituximab plus ESHAP as salvage chemotherapy in relapsed/refractory aggressive histology Non-Hodgkin's Lymphoma [abstract]. Blood. 2003. 102(11):): Abstract. 4875.
Table 1.
Patient characteristics
Table 2.
Response to R-ESHAP compared with ESHAP historical controls




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download