TO THE EDITOR: Development of tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL fusion gene has greatly increased overall survival and major molecular response rates in chronic myeloid leukemia (CML). However, atypical infections such as tuberculosis (TB), hepatitis B virus reactivation, and varicella zoster infection, among others, have been reported after treatment with TKIs [123456]. Furthermore, several preclinical studies have shown that BCR-ABL-targeting TKIs, such as imatinib, dasatinib, and nilotinib, inhibit CD4+ and CD8+ T-cell activity and proliferation [789]. Besides their effects on T cells, recent data have shown that TKIs impair B-cell immune responses in CML through off-target inhibition of kinases important for B-cell signaling [10].
It has been reported that nilotinib does not significantly increase infection compared to imatinib and dasatinib [111], but herein, we report the first case in the literature of TB expressed in the form of atypical pneumonia during nilotinib treatment.
A 45-year-old man was referred to our hospital on account of leukocytosis and splenomegaly. He was diagnosed with chronic phase (CP) CML in March 2011. Treatment was started with imatinib but stopped in May 2011 because of hyperbilirubinemia and pericardial/pleural effusion. Subsequently, imatinib was switched to dasatinib, but after 1 year of dasatinib administration, he developed grade 3–4 pleural effusion and thrombocytopenia. Thus, dasatinib was changed to nilotinib 400 mg twice a day (standard dose) on May 2, 2012.
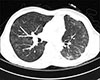
In December 2014, the patient visited the hospital on account of cough, fever, and dyspnea on exertion that had worsened over the prior 2 weeks. Computed tomography (CT) scan of the chest showed diffuse subtle ground glass opacities in both hemithoraces, which was suspicious of atypical pneumonia such as viral infection, pneumocystis pneumonia, miliary TB, or drug-induced pneumonitis (Fig. 1). There was no other specific finding in the lung parenchyma, no lymphadenopathy, and the amount of pleural effusion observed was similar to that observed on the CT scan taken 2 years prior to the event, which had been caused by dasatinib. The initial sputum acid-fast bacilli (AFB) smear stain yielded negative findings, but the interferon-gamma release assay (IGRA) results were positive although the patient had no history of TB. Serum cytomegalovirus and Epstein-Barr virus real-time polymerase chain reaction (PCR) results, and consecutive blood and sputum culture results were all negative.
The bronchoalveolar fluid white blood cell count was 200/µL and was lymphocyte-predominant, comprising 62% lymphocytes and 34% macrophages. Bronchoalveolar fluid AFB stain and TB PCR results were negative.
Initially, intravenous methylprednisolone and intravenous piperacillin/sulbactam were administered based on our suspicion of interstitial lung disease and superimposed bacterial pneumonia based on the CT findings. Despite treatment, his symptoms did not improve. Although no organism was detected on any tests, we considered Pneumocystis jiroveci as the causative organism, based on the atypical pattern on the CT scan and the possible lymphopenic condition. After intravenous trimethoprim-sulfamethoxazole (TMP-SMX) administration, the patients' clinical course improved, and he was discharged on oral TMP-SMX. Two weeks after his first visit with respiratory symptoms, the patient visited the emergency department with anorexia, nausea, and vomiting due to oral TMP-SMX. As the follow-up sputum AFB smear stain yielded positive results, the patient was treated with a 9-month anti-tuberculous regimen which consisted of isoniazid, rifampicin, and ethambutol. Subsequently, 6 weeks after the initial sputum AFB culture test, it turned out to be positive, which finally confirmed TB in this patient. As symptoms improved after antituberculosis medication, nilotinib was restarted at a reduced dosage of 200 mg twice a day. However, after rechallenge with nilotinib, drug-induced interstitial lung disease developed, so the drug was finally changed to radotinib.
Follow-up CT scan performed after 2, 4, and 6 months of antituberculosis therapy showed an improvement of ground glass opacities. The AFB stain result was negative on follow up sputum examination after antituberculosis therapy. However, even after 9 months of antituberculosis medication, the patient's respiratory symptoms remained, so the first-line drugs were continued. After 15 months of antituberculosis drugs, the patient presented with hemoptysis. On the follow-up CT scan, worsening of ground glass opacities was observed, and the sputum AFB smear stain results were positive. Therefore, the antituberculosis regimen was changed to isoniazid, rifampicin, amikacin, cycloserine, and levofloxacin (second-line drugs). Two months following the change of antituberculosis treatment, hepatotoxicity occurred, so the antituberculosis drugs were discontinued. However, after cessation of antituberculosis medications, the results of sputum AFB smear stains performed thrice consecutively were all negative.
Currently, the patient has intermittent cough and sputum production, but is relatively stable without significant X-ray changes. He is taking 200 mg of radotinib twice a day in the outpatient clinic. In March 2018, the BCR-ABL/ABL was quantified as 0.01 international scale normalized copy number, measured at a centralized laboratory by real-time quantitative PCR using an M-bcr Fusion Quant kit (QIAGEN, Hilden, Germany), and his CML showed a major molecular response. The changes in BCR-ABL real-time quantitative PCR according to the treatment of CML and antituberculosis medication are shown in Fig. 2.
Nilotinib is a selective BCR-ABL kinase inhibitor that is indicated for the treatment of newly diagnosed adult patients with Philadelphia chromosome-positive (Ph+) CML in CP, and the treatment of CP and accelerated phase Ph+ CML in adult patients resistant to or intolerant to prior therapy including imatinib. Treatment-free remissions are actively discussed in CML-CP patients, but current guidelines still recommend the continuous use of TKIs. Therefore, complications associated with long-term use of medications should be monitored carefully to ensure patient compliance.
TB may develop after imatinib treatment [26]. This is because imatinib alters T-cell-mediated immune responses [7], raising the possibility of opportunistic infections associated with imatinib therapy. Although nilotinib is also a TKI and has the same mechanism of action as imatinib, no case of TB developing during nilotinib treatment has previously been reported.
Our patient tested positive on IGRA without pulmonary TB findings on previous CT scans, and active TB developed after nilotinib treatment; this could, therefore, be considered a case of latent TB reactivation. Since Korea is an endemic area for TB, it may be controversial to think of it as an infection caused by nilotinib. However, nilotinib may also be a risk factor for TB by inhibiting T cell-mediated immune responses, similar to imatinib [8]. Steroids also inhibit immunity, so we cannot rule out the possibility that pulmonary Tb may be an effect of steroids. However, in our case, as the initial sputum AFB culture obtained before methylprednisolone therapy showed a positive result after 6 weeks of incubation, TB infection was the initial pathogenic event of the pulmonary symptoms and it is less likely that TB was caused by the steroid.
Drug resistance tests of the initial sputum indicated that the TB strain was sensitive to first-line drugs, but respiratory symptoms remained after sufficient treatment. This may have been due to drug-drug interactions (DDIs) between the antituberculosis medications and TKIs.
The patient was switched to second-line drugs due to drug resistance, with acceptable CML and TB treatment outcomes. DDIs between radotinib and antituberculosis medications have not been studied, so further pharmacological studies are required to understand DDIs and to determine the optimum doses of TKIs during antituberculosis therapy.
To the best of our knowledge, this is the first case report of TB developing during nilotinib treatment. In the case described, the clinical manifestations were those of atypical pneumonia, not those of typical pulmonary TB. Furthermore, because it takes at least 6 weeks for culture results for Mycobacterium tuberculosis to be reported, diagnosis and treatment of TB were delayed. As the clinical features of this case were not typical of TB infection, it was difficult to diagnose and properly manage the patient. Thus, when CML patients on nilotinib treatment suffer from atypical pneumonia which is unresponsive to conventional antibiotics, it is important to suspect TB infection and repeat sputum studies even if it is not diagnosed at once. In particular, in areas endemic for TB such as South Korea, the possibility of reactivation of TB in patients receiving treatment with nilotinib should be considered.
Figures and Tables
References
1. Reinwald M, Boch T, Hofmann WK, Buchheidt D. Risk of infectious complications in hemato-oncological patients treated with kinase inhibitors. Biomark Insights. 2016; 10:Suppl 3. 55–68.

2. Daniels JM, Vonk-Noordegraaf A, Janssen JJ, Postmus PE, van Altena R. Tuberculosis complicating imatinib treatment for chronic myeloid leukaemia. Eur Respir J. 2009; 33:670–672.

3. Kang BW, Lee SJ, Moon JH, et al. Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol. 2009; 90:383–387.

4. Mattiuzzi GN, Cortes JE, Talpaz M, et al. Development of Varicella-Zoster virus infection in patients with chronic myelogenous leukemia treated with imatinib mesylate. Clin Cancer Res. 2003; 9:976–980.
5. García-Muñoz R, Galar A, Moreno C, et al. Parvovirus B19 acute infection and a reactivation of cytomegalovirus and herpesvirus 6 in a chronic myeloid leukemia patient during treatment with dasatinib (BMS-354825). Leuk Lymphoma. 2007; 48:2461–2464.

6. Senn L, Kovacsovics T, Tarr PE, Meylan P. Peritoneal tuberculosis after imatinib therapy. Arch Intern Med. 2009; 169:312–313.

7. Seggewiss R, Loré K, Greiner E, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005; 105:2473–2479.

8. Chen J, Schmitt A, Chen B, et al. Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling. J Cell Mol Med. 2008; 12:2107–2118.

9. Fei F, Yu Y, Schmitt A, et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol. 2008; 36:1297–1308.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download