Abstract
Background
Cytomegalovirus (CMV) causes severe diseases in premature infants and immunocompromised hosts, and antiviral therapy is often required for disease control. However, the clinical manifestations and treatment courses for CMV-associated thrombocytopenia in immunocompetent children are unclear.
Methods
Medical records of the children who suffered from thrombocytopenia, and showed positive CMV polymerase chain reaction and CMV-like symptoms were retrospectively analyzed at three university hospitals in Daegu from January 2000 to March 2017. Patients suffering from leukemia, immunodeficiency, and other infections were excluded.
Results
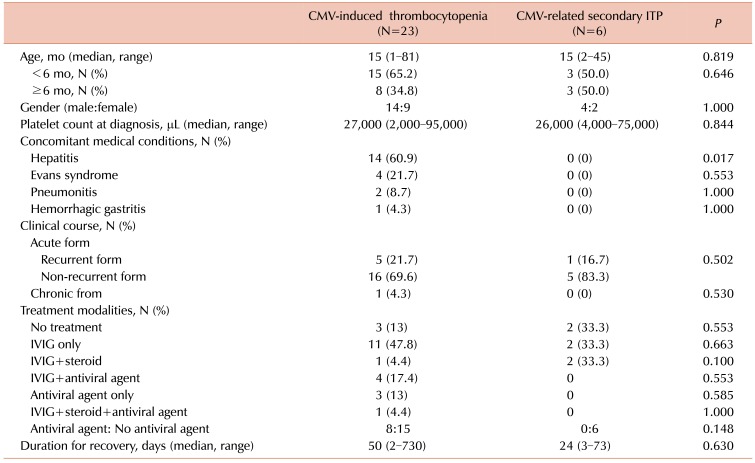
Among 1,065 children with thrombocytopenia, 29 (2.7%) displayed CMV-associated thrombocytopenia. The median age at diagnosis was 15 months and the median platelet count was 26,000/µL. They were classified into the CMV-induced thrombocytopenia (23/29) and CMV-related secondary immune thrombocytopenia (ITP, 6/29) groups. Fourteen subjects had hepatic dysfunction, four had Evans syndrome, two had pneumonitis, and one had gastritis. IVIG was used for 21 patients, and six patients among them showed recurrence, for whom IVIG or antiviral therapy was used. All, except one, recurrent or chronic cases belonged to the CMV-induced thrombocytopenia group. Antiviral therapy was used more frequently for the CMV-induced thrombocytopenia group (8/23, 34.8%) than for the CMV-related secondary ITP group (0/6); however, the results were not statistically significant (P=0.148).
Cytomegalovirus (CMV) causes congenital infections in premature infants and severe diseases in immunosuppressed patients. In immunocompetent patients, the most common form of CMV infection is asymptomatic or benign and self-limited [123]; however, CMV infection is gaining recognition as an uncommon etiology in these patients with severe or persistent thrombocytopenia [4567]. Viruses or bacteria may cause thrombocytopenia via 2 potential mechanisms. First, through direct cytopathic effect by infecting the megakaryocytes, and second, through molecular mimicry or an immune-mediated effect. The former is called CMV-induced thrombocytopenia, while the latter is called CMV-related secondary immune thrombocytopenia (ITP) [789].
Several cases of thrombocytopenia caused by CMV infection in immunocompetent adults or children have been reported [456781011121314]. Further, the importance of studying and understanding CMV infection in immunocompetent children is being emphasized because some of them can result in hepatitis, anemia, thrombocytopenia, neurodevelopmental problems, hearing loss, or growth retardation [1015]. However, in Korea, the frequency and clinical manifestations of CMV infection in immunocompetent children with thrombocytopenia after neonatal period remains unclear. Thus, we reviewed the clinical characteristics and treatment courses for such patients by conducting a multicenter retrospective study.
Children >1 month and <15 years of age with thrombocytopenia and positive CMV polymerase chain reaction (PCR) being treated at Keimyung University Dongsan Medical Center, Kyungpook National University Hospital, and Yeungnam University Medical Center in Daegu from January 2000 to March 2017 were included. Medical records of patients from all three institutes were retrospectively reviewed. This study targeted the previously healthy and immunocompetent children. Patients with primary immunodeficiency, leukemia, inherited bone marrow failure syndrome, or acquired immune deficiency syndrome were excluded from the final analysis. Additionally, patients suffering from non-CMV infections were also excluded.
Thrombocytopenia was defined as the condition in which the platelet count was <100,000/µL. When the platelet count recovered to the normal level (>150,000/µL) within 3–6 months after diagnosis, it was defined as the acute form of thrombocytopenia [161718]. If thrombocytopenia lasted for more than 6–12 months, it was defined as the chronic form. In this study, the recurrent form was defined as follows: thrombocytopenia recovered to the normal level but subsequently dropped to <100,000/µL, regardless of the time interval.
CMV infection was defined as the detection of virus by quantitative or qualitative PCR in the blood or urine samples, performed on the day of detection of thrombocytopenia. As mentioned previously, CMV-induced thrombocytopenia caused by direct infection of megakaryocytes by CMV was defined as the state in which the patients showed CMV-positive PCR and CMV infection-like symptoms (fever, elevated transaminases, jaundice, hepatomegaly, splenomegaly, pneumonitis, gastritis, or myalgia) at the time of thrombocytopenia diagnosis [479]. While, CMV-related secondary ITP caused by immunologic reactions after recent CMV infection was defined as the state in which the patients displayed CMV-like symptoms a few weeks before the diagnosis of thrombocytopenia [479]. Altogether, in the present study, both of them referred to as CMV-associated thrombocytopenia.
Statistical analysis was performed using the SPSS ver. 21.0 software (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U-test was used for comparing variables between the subject groups. The chi-square test was used for ratio comparison between groups. A P-value <0.05 was considered to be statistically significant.
Patients were tested for CMV infection using serological CMV tests (IgM and IgG), viral culture, CMV antigenemia, qualitative PCR, and real-time (or quantitative) PCR for blood or urine samples. CMV IgM and CMV IgG were detected in sera samples using an enzyme immunoassay (ARCHITECT CMV IgG Assay; Abbott Ireland Diagnostic Division, Sligo, Ireland). For detecting CMV in urine, urine samples were collected and cultured using the shell vial culture method (Chemicon, Temecula, CA, USA). For CMV PCR, DNA was extracted from EDTA-treated blood samples using the Prepito Viral NA/gDNA kit (PerkinElmer Chemigen Technogie GmbH, Baesweiler, Germany), amplified using the Amplisens CMV-FEP PCR kit (Central Research Institute of Epidemiology, Moscow, Russia), and detected using fluorescence spectrometer (BioSewoom, Seoul, South Korea).
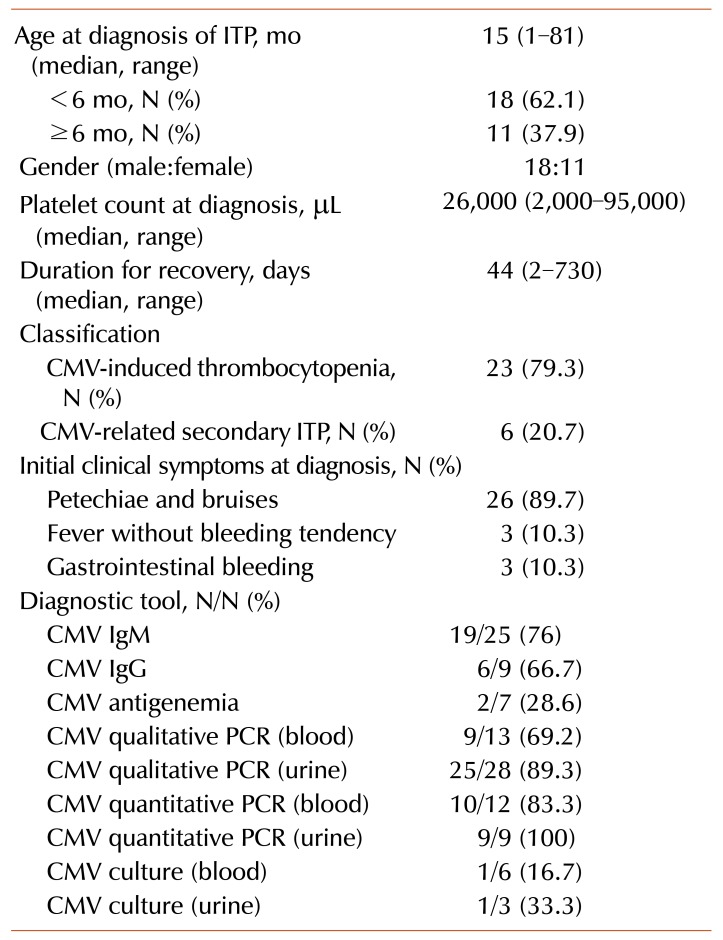
Data for a total of 1,065 children with thrombocytopenia were reviewed. Out of these, 31 patients showed CMV-positive PCR, CMV-like symptoms, and absence of other infections. The tests for respiratory virus, toxoplasma, syphilis, rubella virus, herpes simplex virus, hepatitis virus, and human immunodeficiency virus were negative. Patients did not previously receive blood transfusion. Among the 31 patients, 2 patients with other diseases [Wiskott-Aldrich syndrome (1/31) and juvenile myelomonocytic leukemia (1/31)] were excluded from the analysis. Finally, 29 immunocompetent patients with CMV-associated thrombocytopenia (2.7%) were analyzed. The patients were classified into CMV-induced thrombocytopenia (23/29) and CMV-related secondary ITP (6/29) groups. The baseline characteristics, clinical manifestations, and diagnostic tools of CMV in these patients have been described in Table 1.
Bone marrow analysis was performed for 4 subjects. Of these, 3 belonged to the CMV-induced thrombocytopenia group and 1 belonged to the CMV-related secondary ITP group. The results showed that 1 subject had hypocellular marrow with mild suppression of megakaryopoiesis, while the other 3 subjects, including one CMV-related secondary ITP patient, had normocellular marrow with suppression of megakaryopoiesis. An increase in the aminotransferase levels was evident in 11 patients, while 4 patients showed hyperbilirubinemia, and 1 patient showed increased aminotransferase and bilirubin levels. Two patients showed CMV pneumonitis, confirmed using X-ray of the chest. One patient with hematemesis tested positive for an immunochemistry test for CMV performed using endoscopic biopsy and was diagnosed with CMV gastritis. Audiometric system tests were performed for 6 patients. The results for all 6 patients were normal. Ophthalmological testing was performed for 6 patients. Choriorentinitis was not observed in any patient. Brain imaging analyses were performed for 5 patients (ultrasonogram, 3 patients; magnetic resonance imaging, 2 patients). Intracranial calcification was not observed in any patient.
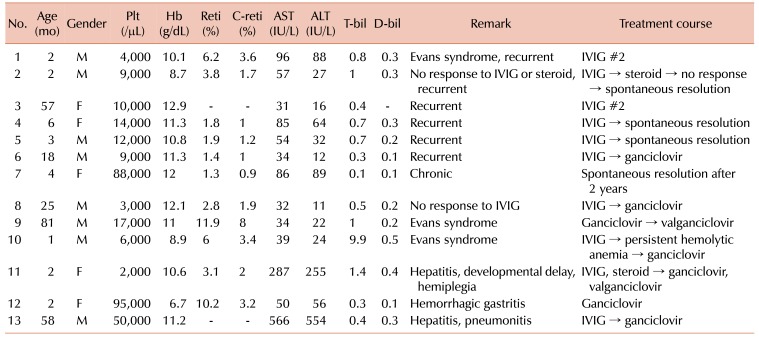
Twenty-eight patients were the acute form (recurrent form, patients 1–6 in Table 2), while 1 patient was the chronic form. All patients, except one (patient 2 in Table 2), showing the recurrent and chronic forms belonged to the CMV-induced thrombocytopenia group. The disease in 5 patients was spontaneously resolved without any treatment. The most frequently prescribed medication was IVIG (prescribed for 21 patients). Corticosteroids were prescribed for 4 patients, and antiviral agents were prescribed for 8 patients. These medications were either used singly or in combination. The disease in all patients was resolved with or without medication.
Detailed description and comparison of clinical treatment courses between the CMV-induced thrombocytopenia group and CMV-related secondary ITP group have been shown in Table 3. Antiviral therapy was used more frequently for the CMV-induced thrombocytopenia group (8/23, 34.8%) than for the CMV-related secondary ITP group (0/6, 0%); however, the difference between them was not statistically significant (P=0.148). The detailed laboratory findings and treatment courses for children who showed complicated clinical manifestations, such as those showing recurrent or chronic forms, those who were refractory to initial therapy, or those who were managed by further treatment other than IVIG have been described in Table 2. All patients, except one (patient 2), listed in Table 2 belonged to the CMV-induced thrombocytopenia group. One patient with chronic ITP (patient 7 in Table 2), belonging to the CMV-induced thrombocytopenia group, showed thrombocytopenia (88,000/µL) at the time of initial diagnosis. This patient displayed several bruises in spite of relatively high level of platelets compared to the general ITP patients. Her thrombocytopenia was prolonged (2 yr), but was spontaneously resolved without any medication.
Three patients (patients 6, 8, and 9 in Table 2) were analyzed for evaluating the correlation between the platelet count in blood and CMV titer in urine samples. A negative correlation was observed between the platelet count and CMV PCR titer, following antiviral treatment (Fig. 1). Patient 6 (Fig. 1A) was initially admitted for thrombocytopenia (platelet 9,000/µL) and multiple severe petechiae/bruises, and showed positive CMV IgM and CMV PCR tests for the urine sample (1,742 copies/mL). After treatment with IVIG (2 g/kg body weight), the platelet count increased to 227,000/µL and the patient was discharged. However, subsequently, his platelet count decreased to 91,000/µL and several petechiae/bruises redeveloped, despite a high platelet count, along with an increased urinary CMV PCR titer (12,834 copies/mL). After treatment with ganciclovir, the CMV PCR titer in urine decreased and the disease resolved without recurrence or bleeding. Initially, patient 8 (Fig. 1B) showed thrombocytopenia (platelet 3,000/µL) and positive CMV PCR (6,315 copies/mL). The disease was refractory to IVIG treatment (2 g/kg) and CMV infection was persistent (6,315 copies/mL→7,277 copies/mL→4,941 copies/mL). After simultaneous treatment with ganciclovir and IVIG, the disease resolved without recurrence and CMV PCR was negative. Patient 9 (Fig. 1C) suffered from Evans syndrome and was initially treated with an antiviral agent. His platelet count was 17,000/µL and CMV PCR showed 1,185 copies/mL. After treatment with ganciclovir and valganciclovir, the platelet count increased to 227,000/µL and CMV PCR showed <100 copies/mL.
Generally, ITP is not very rare. The estimated prevalence of ITP is about 5 cases per 100,000 children [19]. Primary ITP is defined as isolated thrombocytopenia in the absence of other causes. Secondary ITP is defined as any form of thrombocytopenia other than primary, and includes systemic lupus erythematosus, infection, or lymphoproliferative disorders [19]. ITP is believed to be caused by antiplatelet autoantibodies, which potentially destroy platelets and damage megakaryocytes and/or decrease platelet production in the bone marrow [1719]. Several suggestions explaining the potential relationship between CMV infection and thrombocytopenia have been proposed [7]. These include molecular mimicry of host proteins by viral antigens [14], immune dysregulation resulting in increased platelet clearance by the reticuloendothelial system [1719], direct infection of megakaryocytes [2021], production of hematopoietic inhibitory cytokines by the CMV-infected leucocytes and stromal cells of the bone marrow [11], suppression of hematopoiesis of human progenitor cells [1], production of dysfunctional platelets, and induction of vascular diseases [7].
In several cases of CMV-associated thrombocytopenia, thrombocytopenia resolves after the first choice, IVIG [1012]. This suggests a general hypothesis for childhood ITP after viral infections or vaccination. CMV can directly infect megakaryocytes in the bone marrow, thereby decreasing platelet production [2021]. This seems to be the best explanation for some patients who are refractory to conventional ITP therapies, including the use of IVIG and steroids, and who are subsequently treated with antiviral therapy [467]. CMV also causes the megakaryocytes to produce dysfunctional platelets and evokes vasculopathy, which induces severe bleeding symptoms, such as ICH, despite a relatively high platelet count compared to that in the general ITP patients [7]. In our study, 2 children belonging to the CMV-induced thrombocytopenia group (patients 6 and 7 in Table 2) also showed bleeding and their platelet count was 80,000–90,000/µL.
CMV infection shows vertical transmission from mothers to infants via perinatal infection (due to intrapartum transfer) or infected breast milk [22]. Upon infection, the virus remains dormant within the body for a long time. Severe impairment of the immune system or certain diseases reactivate CMV from its latent state. CMV is shed in the body fluids and can be found in the urine, blood, saliva, vaginal fluid, semen, tear, breast milk, and stool samples [22]. Close contact with an infected person, such as in group care settings, neonatal intensive care units, or crowded domestic environments, sometimes causes secondary or community-acquired infections [2324]. Congenital CMV infection is diagnosed by isolating the virus from infants within the first 3 weeks of their birth. After three weeks, it becomes difficult to differentiate whether CMV infection was acquired congenitally or perinatally in infants, unless clinical features of the former such as chorioretinitis, intracranial calcification, microcephaly, and hearing loss are present [22]. In this study, 18 CMV-associated thrombocytopenia patients were less than 6 months old and were never exposed to a day care center. Thus, we hypothesize that CMV infection may be perinatal or transmitted via breast feeding. The remaining 11 patients, who were more than 6 months old and were exposed to day care centers, were considered to have community-acquired secondary infection.
The incidence rate of primary CMV infection is 0.2–2.4% of all live births [22]. After the neonatal period, the incidence of CMV infection varies according to race, economic status, and environmental factors. One study showed that one-third of Pakistani children had CMV infection by the age of 12 months, compared to only 9% of white British children [25]. In another study, most preschool children (>90%) in South America, East Asia, and India were CMV seropositive, in contrast, seroepidemiologic surveys in Great Britain and in USA have found that less than 20% of children of similar age were seropositive [26]. By National Health and Nutrition Examination Survey, 1988–2004 showed that the overall age-adjusted CMV seroprevalence in the USA was about 50% [26]. When analyzing the distribution of CMV IgG positive individuals by age, around 65% of children were shown to have antibodies, and then increased with age, reaching 80% in the 30s and >90% in the 60s [27]. According to the one study about thrombocytopenia patients, 11% children and 4% adults being treated for the management of thrombocytopenia had CMV-positive urine samples [728]. However, in Korea, the current epidemiology of CMV-infected children or proportion of CMV-positive immunocompetent children having thrombocytopenia is not clear. We suggest the frequency/prevalence of CMV-associated thrombocytopenia in this study (2.7%) was underestimated. Because not every thrombocytopenia children tested for CMV PCR, and we only selected the patients with CMV PCR positivity in this study.
Several diagnostic methods for detecting CMV infection are available. Serological tests for detecting the CMV IgM and IgG antibodies are commonly used; however, these tests have some limitations [29]. CMV infection cannot be completely ruled out based on negative CMV IgM test results because the antibody reaction differs between individuals and during the second serological test performed, 2 to 7 days later, seroconversion can occur. Considering the duration for seroconversion, measurement of CMV IgG in samples collected 1 to 3 months apart can be used for diagnosing primary infection. Further, in infants, a positive CMV IgG test potentially reflects transplacentally-acquired maternal antibodies which can persist up to 18 months of age [2230]. Therefore, serological testing has limitations for the early diagnosis of CMV. Qualitative PCR is also used for detecting CMV DNA in blood and tissue samples and is more sensitive than serological tests. However, it is difficult to differentiate latent infection from apparent infection by qualitative PCR, which has low specificity and low-positive predictive rates [29]. On the other hand, quantitation of CMV DNA by real-time PCR, at the exponential phase of viral growth, is fast and accurate. Thus, it is a good diagnostic tool for early diagnosis of CMV infection for initiating timely treatment [29]. Recent studies, including the present study, are using CMV PCR to confirm CMV infection [6713].
Childhood ITP is usually acute, with a recovery rate exceeding 70% [1617]. If ITP persists, it typically responds to ITP-specific therapies, such as IVIG or corticosteroids. IVIG increases the platelet count in more than 80% children [18]. Corticosteroids are also effective and about 75% patients respond to this therapy, although the response is highly dose-dependent [18]. In case of secondary ITP, due to human immunodeficiency virus, Helicobacter pylori, or hepatitis C virus, optimum management requires control of the underlying infection. IVIG is also effective in increasing platelet counts in such cases; however, in some cases, the responses are transient. This is because IVIG only blocks the destruction of platelets, but cannot eradicate the pathogen or increase the production of platelets from the bone marrow. Thus, sometimes, control of the underlying infection is important, following which, standard ITP therapies regain their efficacy [6]. However, exact guidelines for treating CMV-associated thrombocytopenia in immunocompetent hosts, particularly the necessity of antiviral therapy, are unavailable. This is because, in many cases, CMV does not cause severe infections in immunocompetent people [45121428].
In this study, about two-thirds of CMV-associated thrombocytopenia patients showed a simple treatment course and did not need antiviral therapy. However, about one-third patients showed complex diseases (Table 2). About 34.8% patients in the CMV-induced thrombocytopenia group (8/23) required antiviral therapy for disease control. Interestingly, the platelet count was negatively correlated with the serum CMV DNA level in CMV-associated thrombocytopenia patients, and immunosuppression with steroids increased the amount of CMV DNA and decreased platelet levels [6712]. We also observed a negative correlation between the platelet count and the urinary CMV titer during antiviral therapy in 3 CMV-induced thrombocytopenia children (Fig. 1). Thus, we suggest that IVIG should be used as the first line of treatment for CMV-related secondary ITP. Antiviral agents can be considered in cases of CMV-induced thrombocytopenia involving a complex disease course, recurrent or refractory thrombocytopenia post-IVIG therapy, concomitant Evans syndrome, hepatitis, pneumonitis, or gastritis. Antiviral therapy was suggested to be effective and safe for immunocompetent children with CMV-induced thrombocytopenia [9].
In the present study, the prognosis of CMV-associated thrombocytopenia in immunocompetent children was favorable and thrombocytopenia was resolved in all cases. However, in these children, other aspects, such as neuromotor developmental delay, epilepsy, hearing loss, and growth retardation, can coexist along with CMV infection [1015]. The aforementioned points are the limitations of this study. Neurological imaging, and audiometry and neurodevelopmental evaluations were not performed for all patients. The patients diagnosed with CMV-associated thrombocytopenia should also receive thorough neurological and developmental examinations by clinicians [1015].
In conclusion, CMV is a rare and unique pathogen causing childhood thrombocytopenia, even in immunocompetent subjects or during the postneonatal period. Antiviral therapy is required in about one-third cases. CMV-induced thrombocytopenia is more complex than CMV-related secondary ITP. Further research is needed to establish the general treatment guidelines for CMV-associated thrombocytopenia in immunocompetent hosts.
References
1. Arruda VR, Rossi CL, Nogueira E, Annicchino-Bizzacchi JM, Costa FF, Costa SC. Cytomegalovirus infection as cause of severe thrombocytopenia in a nonimmunosuppressed patient. Acta Haematol. 1997; 98:228–230. PMID: 9401503.

2. Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992; 11:93–99. PMID: 1311066.
3. Mutter W, Reddehase MJ, Busch FW, Bühring HJ, Koszinowski UH. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988; 167:1645–1658. PMID: 2896757.

4. Shrestha R, Rondelli D, Sherpa MT. Cytomegalovirus: a possible cause of persistent refractory immune thrombocytopenic purpura. JAIM. 2014; 3:42–45.

5. Shimanovsky A, Patel D, Wasser J. Refractory immune thrombocytopenic purpura and cytomegalovirus infection: a call for a change in the current guidelines. Mediterr J Hematol Infect Dis. 2016; 8:e2016010. PMID: 26740871.

6. Flores-Chang BS, Arias-Morales CE, Wadskier FG, Gupta S, Stoicea N. Immune thrombocytopenic purpura secondary to cytomegalovirus infection: a case report. Front Med (Lausanne). 2015; 2:79. PMID: 26579523.

7. DiMaggio D, Anderson A, Bussel JB. Cytomegalovirus can make immune thrombocytopenic purpura refractory. Br J Haematol. 2009; 146:104–112. PMID: 19438507.

8. Papagianni A, Economou M, Tsoutsou E, Athanassiou-Metaxa M. CMV-related immune thrombocytopenic purpura or CMV-induced thrombocytopenia? Br J Haematol. 2010; 149:454–455. PMID: 20096016.

9. Avila-Agüero ML, Paris MM, Alfaro W, Avila-Agüero CR, Faingezicht I. Ganciclovir therapy in cytomegalovirus (CMV) infection in immunocompetent pediatric patients. Int J Infect Dis. 2003; 7:278–281. PMID: 14656419.

10. Kang JW, Kim GN, Kim SY, et al. Clinical and radiologic evaluation of cytomegalovirus-induced thrombocytopenia in infants between 1 and 6 months of age. Korean J Hematol. 2010; 45:29–35. PMID: 21120160.

11. Miyahara M, Shimamoto Y, Yamada H, Shibata K, Matsuzaki M, Ono K. Cytomegalovirus-associated myelodysplasia and thrombocytopenia in an immunocompetent adult. Ann Hematol. 1997; 74:99–101. PMID: 9063381.

12. Palumbo E, Bonora G. Cytomegalovirus-induced thrombocytopenia in an immunocompetent child. Infect Dis Clin Pract. 2008; 16:54–56.

13. Arruda VR, Oliveira GB, Annichino-Bizzacchi JM, Durante P, Costa FF, Costa SC. CMV-DNA detection in patients with thrombocytopenia. Sao Paulo Med J. 2002; 120:62. PMID: 11994776.

14. Wright JG. Severe thrombocytopenia secondary to asymptomatic cytomegalovirus infection in an immunocompetent host. J Clin Pathol. 1992; 45:1037–1038. PMID: 1333493.

15. Çelikel E, Tezer H, Kanik-Yuksek S, Gülhan B, Ozkaya-Parlakay A, Yaralı N. Evaluation of 98 immunocompetent children with cytomegalovirus infection: importance of neurodevelopmental follow-up. Eur J Pediatr. 2015; 174:1101–1107. PMID: 25762027.

16. Shim YJ, Kim UH, Suh JK, Lee KS. Natural course of childhood chronic immune thrombocytopenia using the revised terminology and definitions of the international working group: a single center experience. Blood Res. 2014; 49:187–191. PMID: 25325039.

17. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009; 113:2386–2393. PMID: 19005182.

19. Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013; 27:495–520. PMID: 23714309.

20. Crapnell K, Zanjani ED, Chaudhuri A, Ascensao JL, St Jeor S, Maciejewski JP. In vitro infection of megakaryocytes and their precursors by human cytomegalovirus. Blood. 2000; 95:487–493. PMID: 10627453.

21. Xiao Y, Lin W, Liu Q, Jin R, Fei H. Direct infection of colony forming unit-megakaryocyte by human cytomegalovirus contributes the pathogenesis of idiopathic thrombocytopenic purpura. J Huazhong Univ Sci Technolog Med Sci. 2006; 26:555–557. PMID: 17219966.

23. Demmler GJ, Yow MD, Spector SA, et al. Nosocomial cytomegalovirus infections within two hospitals caring for infants and children. J Infect Dis. 1987; 156:9–16. PMID: 3036963.

24. Aitken C, Jeffries DJ. Nosocomial spread of viral disease. Clin Microbiol Rev. 2001; 14:528–546. PMID: 11432812.

25. Pembrey L, Waiblinger D, Griffiths P, Patel M, Azad R, Wright J. Cytomegalovirus, Epstein-Barr virus and varicella zoster virus infection in the first two years of life: a cohort study in Bradford, UK. BMC Infect Dis. 2017; 17:220. PMID: 28320319.

26. Elzouki AY, Harfi HA, Nazer H, Oh W, Stapleton FB, Whitley RJ. Textbook of clinical pediatrics. 2nd ed. New York, NY: Springer-Verlag;2012. p. 1145–1161.
27. Lopo S, Vinagre E, Palminha P, Paixao MT, Nogueira P, Freitas MG. Seroprevalence to cytomegalovirus in the Portuguese population, 2002–2003. Euro Surveill. 2011; 16:19896. PMID: 21722611.

28. Levy AS, Bussel J. Immune thrombocytopenic purpura: investigation of the role of cytomegalovirus infection. Br J Haematol. 2004; 126:622–623. PMID: 15287958.

29. Jeong S, Kim YJ, Bae IK, Kim MJ, Jeong SH. Comparison cytomegalovirus qualitative assay using real-time PCR and conventional PCR. Ann Clin Microbiol. 2013; 16:19–24.

30. Berardi A, Rossi C, Fiorini V, et al. Severe acquired cytomegalovirus infection in a full-term, formula-fed infant: case report. BMC Pediatr. 2011; 11:52. PMID: 21645352.

Fig. 1
Negative correlation between platelet count and urinary real-time quantitative CMV PCR titer during anti-viral treatment in CMV ITP children.
Abbreviations: CMV, cytomegalovirus; IVIG, intravenous immunoglobulin; PCR, polymerase chain reaction.
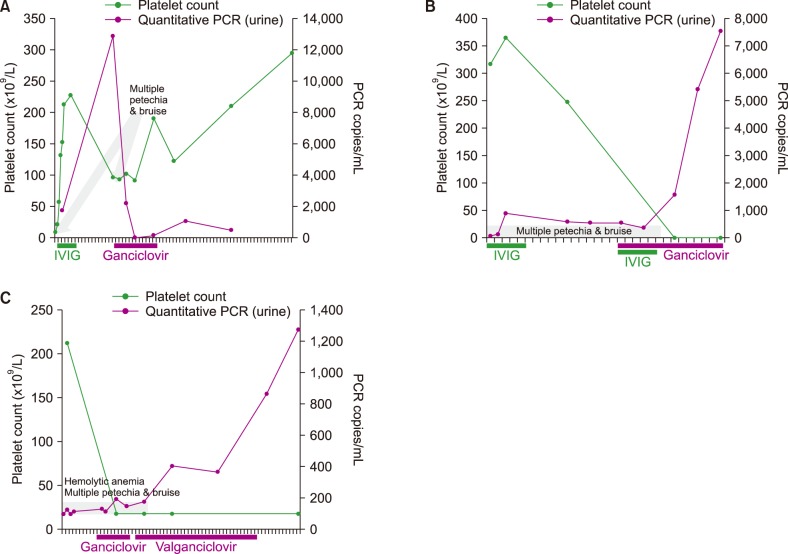
Table 1
The baseline characteristics of 29 CMV-associated thrombocytopenia children after neonatal period.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download