Abstract
Background
Autoimmune hemolytic anemia (AIHA) is a less recognized, potentially fatal condition. There is a scarcity of data on clinicoserological characteristics and response to therapy concerning this disease from South India.
Methods
Data for 33 patients with primary AIHA recorded from July 2009 to June 2015 were retrospectively analyzed for clinical presentation, response to frontline therapy, durability of response, time to next treatment (TTNT), and response to second-line agents.
Results
The median follow-up period was 50 months. Among 33 patients, 48% of the cases were warm autoimmune hemolytic anemia (WAIHA), 46% were cold agglutinin disease (CAD), and 6% were atypical. Three-fourth of patients had severe anemia (<8 g/dL hemoglobin [Hb]) at onset; younger patients (age <40 yr) had more severe anemia. All of the patients who required treatment received oral prednisolone at 1.5 mg/kg/d as a frontline therapy, and the response rate was 90% (62% complete response [CR] and 28% partial response [PR]). The overall response to corticosteroids in WAIHA and CAD was 87% and 92%, respectively. The median corticosteroid duration was 14 months, and 50% of the patients required second-line agents. Fourteen patients received azathioprine as a second-line agent, and 11 of these patients responded well, with half of them not requiring a third agent. Four patients developed severe infections (pneumonia, sepsis, and soft tissue abscess) and two had life-threatening venous thrombosis. One case of death was recorded.
Autoimmune hemolytic anemia (AIHA) is a rare, potentially fatal condition that often requires prolonged immunosuppressive therapy. In the West, its incidence is 1–3 per 105 per year and its prevalence is 17:100,000 [1]. There is a lack of data about the incidence of AIHA in India. This disease is often not recognized and diagnosis is delayed. Autoantibodies produced against red cell antigens cause hemolytic anemia in this condition. This may be a primary (idiopathic) process or a secondary process caused by other diseases such as autoimmune disorders, lymphoproliferative disorders, infections, or tumors [23]. Presentation is varied, ranging from insidious onset of anemia with hyperbilirubinemia progressing over months to acute fulminant hemolysis occurring within hours to days and leading to a sudden drop in hemoglobin. The diagnosis is made using the direct antiglobulin test (DAT) with polyspecific antiglobulin reagents. Two serological types of the disease, warm autoimmune hemolytic anemia and cold agglutinin disease, are recognized based on the thermal range of the autoantibodies. In warm autoimmune hemolytic anemia (WAIHA), the DAT usually yields positive results with anti-immunoglobulin G (anti-IgG) antisera and, in CAD, the DAT yields positive results with anti-C3d antisera and indicates the presence of high titer cold agglutinins. The autoantibodies produced in CAD usually belong to the IgM subclass. In some proportion of cases, the thermal range of antibodies may be substantially wide enough to cause hemolysis in both warm and cold conditions. These cases are detected via the coexistence of warm antibodies and high titer cold agglutinins and are designated as mixed type AIHA. Even with the availability of sensitive techniques such as the microalbumin test, flow cytometry, and mitogen-stimulated DAT [45], in one-tenth of AIHA cases, the DAT can demonstrate negative results, and these cases are designated as atypical AIHA [1]. Because of a lack of prospective clinical trials [6], the treatment of AIHA is based on large clinical studies [789], experts' opinions, and individual experience. While the first option for treatment of WAIHA is usually corticosteroids, there is no consensus for the treatment of CAD. The choice of second-line agents in cases of relapsed disease is based on a physician's comfort with various immunosuppressive agents such as azathioprine, cyclosporin, mycophenolate mofetil (MMF), and anti-CD20 monoclonal antibody (rituximab) and splenectomy. There is a lack of data concerning the diagnosis and treatment of AIHA in India. With this background, we decided to study the clinical profile of this disease, its response to frontline and second-line agents, and the occurrence of complications and to correlate this information with the different serological types of the disease.
From July 2009 to June 2015, 33 consecutive patients with primary AIHA who attended the Hematology Clinic of Amrita Institute of Medical Sciences, Kochi, India, which is a tertiary referral center for hematology in South India, were analyzed for clinical presentation, response to frontline therapy, durability of response, TTNT, and response to second-line agents. Patients with AIHA without any autoimmune disorders, lymphoproliferative disorders, malignancies, or infections were included.
We performed the direct Coombs test with gel Coombs cards (Biorad Diamed GmbH, Switzerland) and polyspecific anti-human globulin (AHG) serum containing anti-IgG and C3d. A 0.8% red cell suspension was used for testing as per the manufacturer's instructions. The indirect Coombs test was performed using a 3-cell reagent panel at 37℃. The results were graded from 1+ to 4+. An autocontrol was also performed at 37℃ in the AHG phase using the Coombs card. A positive indirect Coombs test at 37℃ indicated the presence of clinically significant antibodies. A positive result with the autocontrol indicated the presence of autoantibodies.
The cold agglutinin test was performed by adding 2 drops of the patient's serum and one drop of pooled O cells in a test tube. After incubation at 4℃ for 4 hours, agglutination was observed macroscopically. Agglutination with 3+ to 4+ was interpreted as a positive test result, which indicated the presence of cold agglutinins. Those patients who had negative DAT results and no cold agglutinins were considered to have atypical AIHA.
At the time of diagnosis, hemoglobin levels of 10.1–12.0, 8.1–10.0, 6.1–8.0, and <6 g/L were indicative of mild, moderate, severe, and very severe anemia, respectively. The Institutional Ethics Committee approved the study protocol. In accordance with the Declaration of Helsinki, the patients provided informed consent.
Corticosteroids were the frontline treatment for patients who required treatment. Prednisolone was administered at 1.5 mg/kg/d for 3 weeks and then gradually tapered and eventually discontinued over 2–3 years if the patients maintained a response. Patients who did not respond to or relapsed after corticosteroid treatment, were administered another agent such as azathioprine, MMF, cyclophosphamide, cyclosporin, or rituximab either at a low dose of 100 mg/wk for 4 weeks or a standard dose of 500 mg/wk for 4 weeks. In fulminant cases that were unresponsive to corticosteroids, intravenous immunoglobulin (IVIG) was administered at 1 g/kg/d for 2 days. Packed red blood cell (PRBC) transfusion requirements were noted. The responses to therapy were defined as CR (>12 g/dL Hb with no evidence of ongoing hemolysis) and PR (>10 g/dL Hb or >2 g/dL increase in Hb and no transfusion requirements). We investigated the clinical outcomes including the TTNT following steroid treatment at least due to the lack of PR or relapse (decrease in Hb to <10 g/dL), life-threatening infections, thrombotic events, and death.
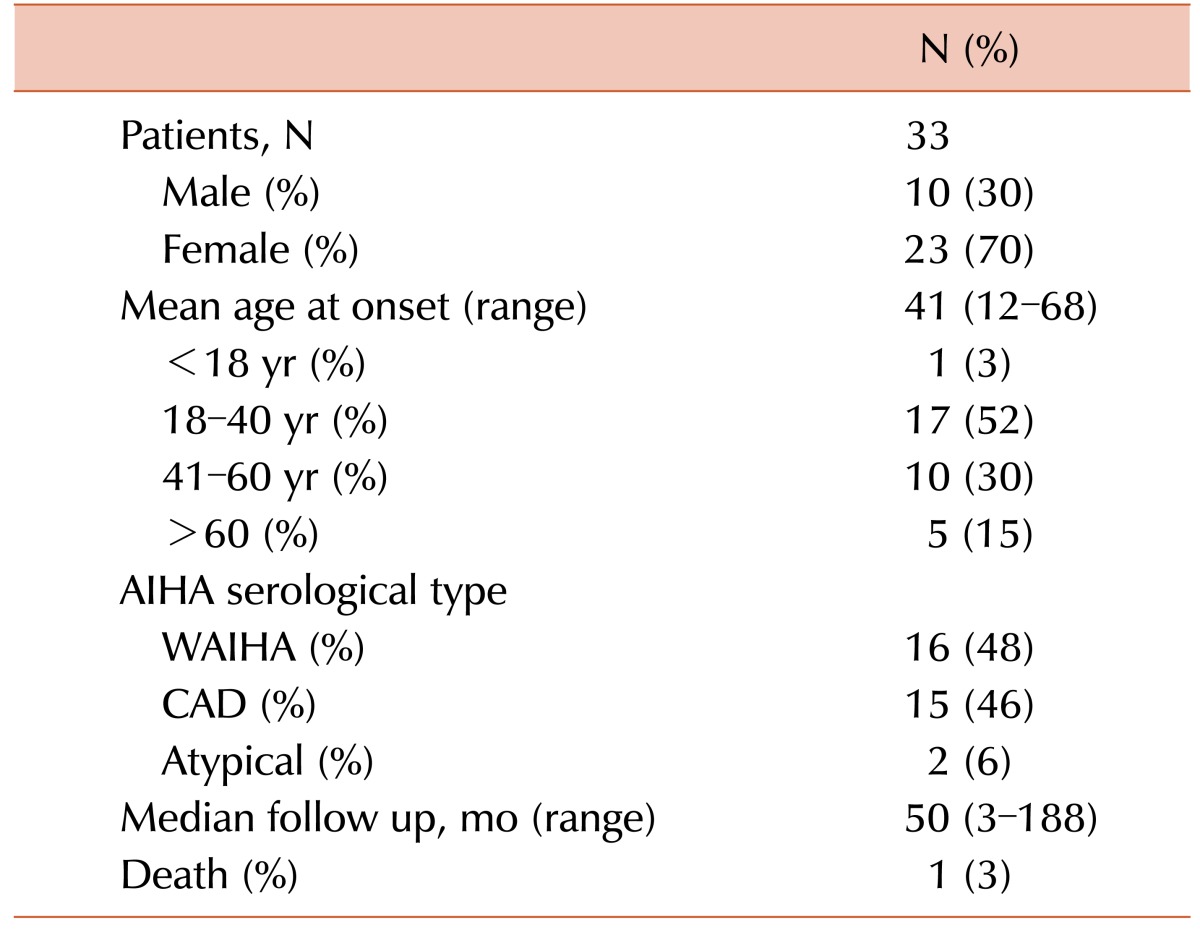
There was nearly an equal number of WAIHA and CAD patients (Table 1). The median age was 41 years (range, 12–68 yr). The male to female ratio was 1:2.3. Two patients had neither positive DAT nor cold agglutinin test results and were diagnosed with atypical AIHA.
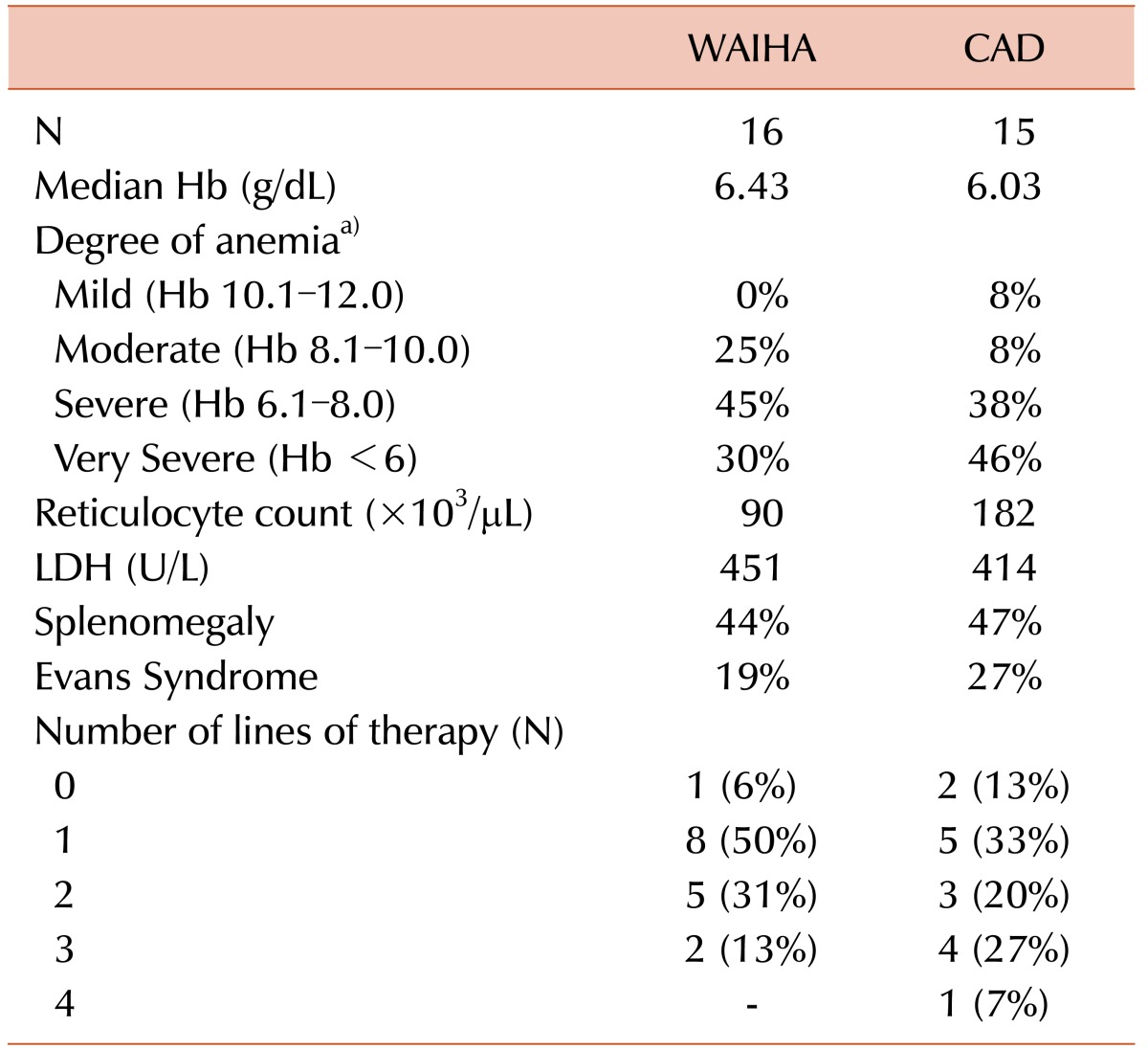
Table 2 compares the laboratory features of WAIHA and CAD. The median Hb was comparable in WAIHA and CAD patients (6.43±1.91 g/dL vs. 6.03±2.01 g/dL, P=0.44). While 46% of CAD cases involved very severe anemia (<6 g/dL Hb) at onset, only 30% of WAIHA cases involved very severe anemia at diagnosis, though this was not statistically significant (P=0.67). The mean Hb level was 8 g/dL (range, 7–9 g/dL) in the two atypical cases. Younger patients had more severe anemia; the median age of those with <6 g/dL Hb was 31 years, while the median age of those with >6 g/dL Hb was 47 years (P=0.019). There was no statistically significant difference in the median lactate dehydrogenase (LDH) level (451±683 U/L vs. 414±212 U/L, P=0.79) and the median reticulocyte count (90±89×103/µL vs. 182±82×103/µL, P=0.52) between WAIHA and CAD cases. The mean reticulocyte count was 151×103/µL and the mean LDH was 370 U/L in the two atypical cases. Splenomegaly was present in 14 (42%) cases, 7 cases each of WAIHA and CAD. Evans syndrome (ES) was diagnosed in 8 patients (24%) either at diagnosis or during relapse; 4 of them were CAD cases, 3 were WAIHA, and 1 was atypical AIHA. In 2 of the WAIHA patients, the disease/relapse was precipitated by pregnancy. The patient with ES relapsed when she became pregnant, while the other patient presented in the postpartum period.
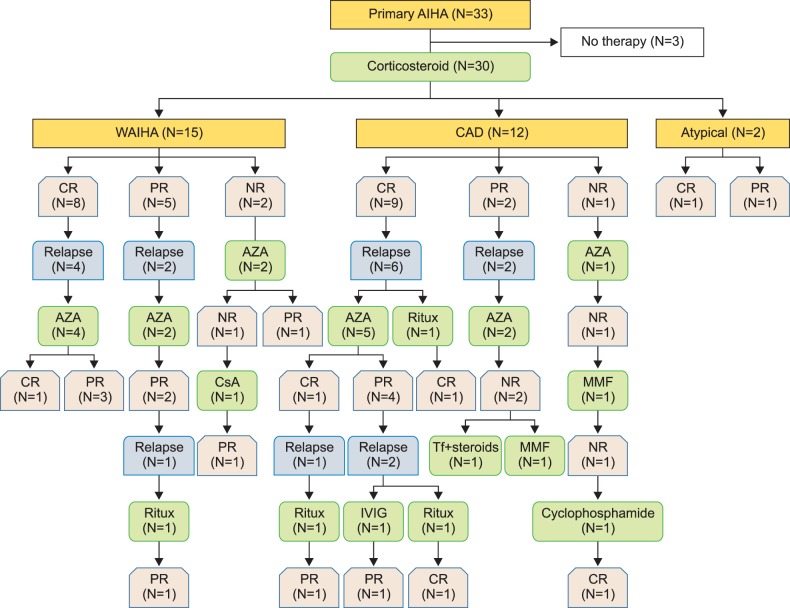
Table 2 shows the number of lines of therapy received by the WAIHA and CAD patients. Fig. 1 illustrates the sequence of the therapies received according to serological type. One CAD patient required 4 lines of therapy. Of the 6 patients requiring 3 lines of therapy, 4 had CAD and 2 had WAIHA. Of the 8 patients who required 2 lines of therapy, 5 had WAIHA and 3 had CAD. Eight patients with WAIHA, 5 with CAD, and both patients with atypical AIHA required only 1 line of therapy. The median follow-up duration was 50 months (range, 3–188 mo).
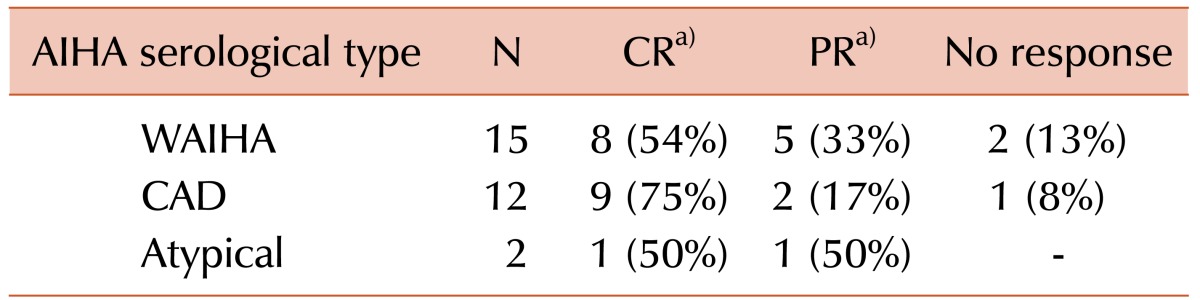
All the 30 patients who required treatment received corticosteroids as the first-line therapy. In 18 of these patients, intravenous corticosteroids (high-dose methylprednisolone or high-dose dexamethasone) were administered for 3–4 days preceding oral prednisolone (1.5 mg/kg/d). Of the 29 evaluable patients, 26 (90%) achieved a response, with 18 (62%) achieving CR and 8 (28%) achieving PR. Three patients (10%) did not achieve any response. Their serological characteristics are shown in Table 3. Though 75% of CAD patients achieved CR versus 54% of WAIHA patients, this was not statistically significant (P=0.27). The median steroid duration was 14 months (range, 1–69 mo). Fifteen of these patients required a second-line agent with a median TTNT of 19 months (range, 5––69 mo).
All but one of the 15 patients received azathioprine as the second-line agent. The remaining patient received low dose rituximab (rituximab LD) of 100 mg weekly for 4 weeks. The median azathioprine duration was 19 months (range, 1–109 mo). The overall response to azathioprine was 79% (11/14 patients) with 64% (9/14 patients) PR and 15% (2/14 patients) CR. The response to azathioprine did not depend on the AIHA serological type. The patient on rituximab LD had CAD and achieved PR.
Of the 7 patients who required a third-line agent, 2 were treated with standard dose rituximab (rituximab SD) and both achieved PR. One of these patients had WAIHA and the other CAD. One CAD patient was started on third-line rituximab LD and achieved CR. Two CAD patients who were started on third-line MMF did not respond to treatment. One WAIHA patient and one CAD patient received cyclosporin and IVIG, respectively, as third-line agents and both achieved PR.
Twenty-one of the 33 patients required PRBC transfusions. Among them, 12 had CAD, 8 had WAIHA, and 1 had atypical AIHA. There was no statistically significant difference in transfusion requirements between WAIHA and CAD cases (P=0.2).
Four of the 33 patients developed serious infections; 1 WAIHA patient and 1 CAD patient developed pneumonia. One of these patients had community-acquired pneumonia while on a very low dose of prednisolone (5 mg on alternate days) and azathioprine and the other had nosocomial pneumonia while in the intensive care unit for treating cerebral venous thrombosis. We did not isolate any organisms from both patients' sputum and blood.
One atypical AIHA patient developed bacteremia and sepsis and died. This was the only death in this series. One CAD patient developed a popliteal abscess while on prednisolone that required surgical drainage.
Two patients had venous thrombotic events; 1 CAD patient had cerebral venous thrombosis and 1 WAIHA patient developed pulmonary embolism. Interestingly, both of these patients had ES.
Because AIHA is a rare disease with varied presentation, serological characteristics, and severity, it is difficult to conduct properly designed prospective studies. As it is a benign disease and thus less 'glamorous' than other hematological disorders such as leukemia and myeloma, hemato/oncologists often disregard this potentially fatal disease. Because of a lack of well-established guidelines and heterogeneity, therapy is a challenge and often prolonged therapy is required. There is dearth of data from South India. We retrospectively analyzed our patients. Given the rarity of the disease, our numbers are considerable, especially as this was a single center study. Similar to a previous, large GIMEMA study [1], we had a predominantly female patient population. However, in contrast to that study, the median age of our population was lower (41 yr) and the majority belonged to the young adult group (18–40 yr). This younger age of onset in the Indian population is in agreement with a previous observation from northern India where the median age was 31 years [10]. However, that study included both primary and secondary AIHA patients. In contrast to previous studies [111213], three-fourth of our patients had severe anemia at presentation with about 42% having reticulocytopenia, probably due to immune-related destruction of reticulocytes and apoptosis of red cell precursors [14]. One interesting observation was that younger patients had a severe onset of disease. The reason for this could be diagnostic delay, as nutritional anemia is the commonest cause of anemia and patients with anemia are often initially treated with hematinics before they are referred to specialists for evaluation. Upfront systematic evaluation of anemia is important as earlier initiation of therapy for AIHA lowers the probability of relapse [23].
We had an equal proportion of WAIHA and CAD patients. This is in contrast to previous reports that had a preponderance of warm cases [23]. Only 6% of our cases were DAT negative atypical AIHA. This is in contrast to most previously reported series, but in agreement with a recent large observational study [1]. We used a more sensitive gel technique rather than conventional tube tests, which explains the low percentage of atypical AIHA. Surprisingly, in these atypical cases, the average Hb was higher than DAT positive cases. One expects a certain diagnostic delay in DAT negative cases and, consequently, a drop in Hb while awaiting the completion of diagnostic work. One possible explanation is that such cases produce a much lower level of autoantibodies than the detection threshold of current techniques and, consequently, the rate of hemolysis is indolent and leads to a slow decline in Hb. In our series, the average Hb was comparable between WAIHA and CAD cases. One expects greater hemolysis and, consequently, more severe anemia in CAD due to IgM-mediated intravascular hemolysis than in WAIHA, which involves IgG-mediated extravascular hemolysis [23].
All of the 30 patients who required treatment were treated with upfront high-dose corticosteroid therapy. Among them, 90% achieved a response irrespective of the serological type. More patients with CAD achieved CR than WAIHA patients, though this was not statistically significant. This contrasts with most previous reports, but is in agreement with a previous report from India [10] where the overall response to corticosteroids was 88% regardless of serological type. Although a large number of patients respond to initial corticosteroid therapy, it is well-established that less than a fifth are cured by corticosteroids alone and maintain persistent complete remission [2315]. Our study agrees with this observation; 13% of the patients who required steroid therapy are in CR and off medications and another 17% are in CR with a low dose of steroids (<15 mg/d prednisolone). The median duration of steroid treatment in this series was 14 months. It has been shown that patients who continue to receive low dose corticosteroids for more than 6 months have a lower chance of relapse than those in whom corticosteroids were discontinued before 6 months [16]. Although recent recommendations advise against corticosteroid therapy for CAD [9], we found a good response with corticosteroids in these patients. It should be noted that we did not differentiate between mixed type AIHA, where antibodies have a large thermal amplitude, and CAD, which, to some extent, may explain the better response seen in CAD cases. However, according to most previous reports, one only expects around 10% of mixed cases and this cannot fully offset the good results we observed in our CAD cases [1].
The small difference between median steroid duration (14 mo) and median TTNT (19 mo) indicates that the majority of patients who are off corticosteroids will eventually relapse and require further agents. The assessment of the efficacy of second-line agents is blurred by the fact that patients are simultaneously on second-line agents and corticosteroids, and it is often not possible to exactly delineate the benefit of the second-line agent. We have had maximum experience with azathioprine in relapsed cases and, in contrast to the findings of a recent large study [1], nearly four-fifths of our patients responded durably regardless of the AIHA type. We have had limited experience with other immunosuppressive agents and hence cannot draw any inference of their effect from this study. Of note, we did not see a response in both patients who received MMF. This is an interesting observation, especially as MMF has been proven to be effective in post-hematopoietic stem cell transplant AIHA [1718]. The extensive use of azathioprine instead of rituximab, which is recommended as a first-line agent for CAD [9], is because rituximab is unaffordable to most in a country where patients pay out of their pocket.
Of the 15 patients who required therapeutic agents beyond steroids, 4 received rituximab therapy; 1 received it as a second-line agent and 3 as a third-line agent. All of these patients achieved at least PR. A response was also observed in patients administered rituximab LD. This is interesting as rituximab LD would substantially save costs. However, according to recent reports, larger prospective studies of rituximab LD are needed before making it a routine clinical practice, especially in CAD cases [61920].
Interestingly, we did not treat a single patient with splenectomy. This possibly indicates the availability of more therapeutic agents and the reluctance of patients to undergo a surgical procedure that has a low but significant risk of post-splenectomy sepsis. In line with the severe presentation, two-thirds of our patients initially required PRBC transfusions. However, all of them were transfusion-independent one week into steroid therapy.
Our series of primary AIHA cases shows that nearly half of our patients required two lines of therapy and one-fifth required a third agent. A younger age was associated with a more severe clinical presentation. There was no difference in response to corticosteroids among WAIHA and CAD cases. In relapsed cases, four-fifths of the cases responded to azathioprine in conjunction with corticosteroids and half of these patients did not require a third agent.
Two patients in our series had venous thrombosis. Of these, 1 patient had a stormy clinical course with pneumonia and required 3 lines of therapy. Both of them did not have anticardiolipin antibodies. An association has been described in AIHA between venous thrombosis and anticardiolipin antibodies [21]. Interestingly, both patients who developed venous thrombosis had ES, which indicates that patients with ES are at a higher risk for thrombotic events than those with AIHA alone. Prothrombotic risk in ES cases has been recognized before [22]. There were 4 infective episodes: 1 was grade 2, 2 were grade 4, and 1 was grade 5. The patient who died had concomitant chronic liver disease and bacteremia and developed severe sepsis.
In conclusion, our study is the first from South India to observe the clinicoserological characteristics of AIHA patients. This is probably the first study from India to examine the effectiveness of second-line agents in AIHA. We found that a younger age of onset is associated with severe disease presentation. We observed an equal proportion of warm and cold cases. We found that contrary to recent reports, cold cases responded well to upfront corticosteroids. Although an observational study is not ideal for making treatment recommendations, we found that azathioprine is an effective second-line agent. With the availability of rituximab and because of the risk of severe post-splenectomy sepsis, the role of splenectomy has considerably decreased. Finally, AIHA is a very heterogeneous disease with a potential for fatal outcome that requires care by physicians experienced in treating these patients.
References
1. Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014; 124:2930–2936. PMID: 25232059.

2. Petz LD, Garratty G. Immune hemolytic anemias. 2nd ed. Philadelphia, PA: Churchill Livingstone;2004.
3. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002; 69:258–271. PMID: 11921020.

4. Barcellini W, Revelli N, Imperiali FG, et al. Comparison of traditional methods and mitogen-stimulated direct antiglobulin test for detection of anti-red blood cell autoimmunity. Int J Hematol. 2010; 91:762–769. PMID: 20454945.

5. Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemias. Haematologica. 2014; 99:1547–1554. PMID: 25271314.

6. Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004; 103:2925–2928. PMID: 15070665.

7. Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010; 116:1831–1838. PMID: 20548093.

8. Crowther M, Chan YL, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011; 118:4036–4040. PMID: 21778343.

9. Berentsen S, Tjønnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012; 26:107–115. PMID: 22330255.

10. Naithani R, Agrawal N, Mahapatra M, Pati H, Kumar R, Choudhary VP. Autoimmune hemolytic anemia in India: clinico-hematological spectrum of 79 cases. Hematology. 2006; 11:73–76. PMID: 16522555.

11. Liesveld JL, Rowe JM, Lichtman MA. Variability of the erythropoietic response in autoimmune hemolytic anemia: analysis of 109 cases. Blood. 1987; 69:820–826. PMID: 3814817.

12. Aladjidi N, Leverger G, Leblanc T, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica. 2011; 96:655–663. PMID: 21228033.

13. Naithani R, Agrawal N, Mahapatra M, Kumar R, Pati HP, Choudhry VP. Autoimmune hemolytic anemia in children. Pediatr Hematol Oncol. 2007; 24:309–315. PMID: 17613874.

14. Van De Loosdrecht AA, Hendriks DW, Blom NR, Smit JW, De Wolf JT, Vellenga E. Excessive apoptosis of bone marrow erythroblasts in a patient with autoimmune haemolytic anaemia with reticulocytopenia. Br J Haematol. 2000; 108:313–315. PMID: 10691861.

15. Kamesaki T, Toyotsuji T, Kajii E. Characterization of direct antiglobulin test-negative autoimmune hemolytic anemia: a study of 154 cases. Am J Hematol. 2013; 88:93–96. PMID: 23169533.

16. Dussadee K, Taka O, Thedsawad A, Wanachiwanawin W. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thai. 2010; 93(Suppl 1):S165–S170. PMID: 20364571.
17. Jaime-Pérez JC, Rodríguez-Martínez M, Gómez-de-León A, Tarín-Arzaga L, Gómez-Almaguer D. Current approaches for the treatment of autoimmune hemolytic anemia. Arch Immunol Ther Exp (Warsz). 2013; 61:385–395. PMID: 23689532.

18. O'Connell N, Goodyer M, Gleeson M, et al. Successful treatment with rituximab and mycophenolate mofetil of refractory autoimmune hemolytic anemia post-hematopoietic stem cell transplant for dyskeratosis congenita due to TINF2 mutation. Pediatr Transplant. 2014; 18:E22–E24. PMID: 24168326.
19. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013; 122:1114–1121. PMID: 23757733.

20. Berentsen S, Randen U, Vågan AM, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010; 116:3180–3184. PMID: 20634373.

21. Hoffman PC. Immune hemolytic anemia--selected topics. Hematology Am Soc Hematol Educ Program. 2009; 80–86. PMID: 20008185.

Fig. 1
Sequence of the therapies and clinical outcomes in the primary autoimmune hemolytic anemia.
Abbreviations: AIHA, autoimmune hemolytic anemia; WAIHA, warm AIHA; CAD, cold agglutinin disease; CR, complete remission; PR, partial remission; NR, no response; AZA, azathioprine; Ritux, rituximab; CsA, cyclosporin; Tf, transfusion; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download