TO THE EDITOR: The involvement of bone marrow (BM) in aggressive non-Hodgkin lymphomas (NHLs) usually indicates systemic dissemination. However, although uncommon, extranodal involvement of the BM as an isolated and unique localization of aggressive NHLs [1] such as anaplastic large cell lymphoma [2] or diffuse large B-cell lymphoma (DLBCL) [3-6], has also been reported. In particular, primary DLBCL of the BM is a rare type of extranodal lymphoma with poor prognosis [1, 4]. Approximately 10 cases of primary DLBCL of the BM have been described thus far [3], and few of them have been reported in individuals of very advanced age. Here, we report a case of primary DLBCL of the BM that was successfully treated using rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP chemotherapy; 3-week standard schedule) in a frail 76-year-old woman.
The patient was being treated for pancytopenia and dyspnea on exertion caused by severe macrocytic anemia, which required multiple red blood cells (RBC) transfusions. Her Eastern Cooperative Oncology Group performance status was 2. In terms of comorbidities, she presented with a long-standing history of hypertension, reduced renal function (creatinine clearance as estimated by the Cockroft-Gault formula was 60 mL/min·1.73 m2), and chronic cerebrovascular disease with slight cognitive and neurological impairment. Her antihypertensive medication consisted of a combination of enalapril and amlodipine. At admission, the patient presented with severe malaise and fatigue. Physical examination revealed pallor and tachycardia, but neither lymphadenopathy nor hepatosplenomegaly was observed. Morphological examination of peripheral blood smears showed marked thrombocytopenia with prominent erythrocyte and platelet anisopoikilocytosis; however, no other abnormalities were detected. Although rare circulating neutrophils were present, no immature or atypical cells were observed.
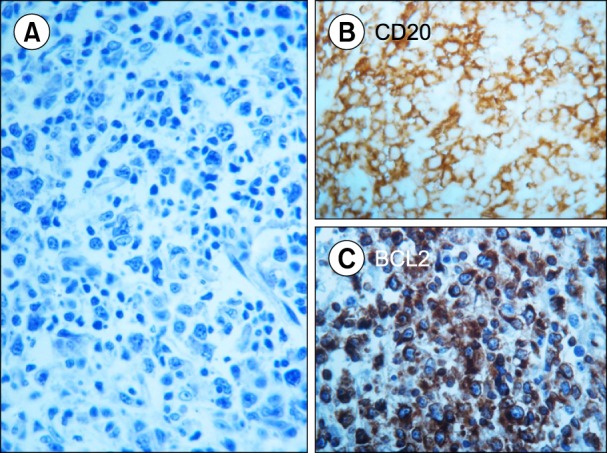
Myelodysplastic syndrome (MDS) was suspected, and a comprehensive work-up was performed. BM aspiration revealed a dry tap; examination of the BM specimen by trephine biopsy showed involvement of large abnormal lymphoid cells (Fig. 1) and fibrosis. A standard radiological work-up found no other suspected disease localizations. 18F-fluorodeoxyglucose positron emission tomography (PET) [7] revealed disseminated BM with diffuse uptake with no evidence of disease involvement of other sites. The patient was diagnosed with primary DLBCL. A comprehensive laboratory evaluation showed high levels of lactate dehydrogenase but no other remarkable abnormalities. The age-adjusted International Prognostic Index was 3 (high risk).
Although the prognosis of this type of NHL is poor and requires an aggressive approach, including high-dose chemotherapy with autologous stem cell transplantation (ASCT), which would normally be indicated [1, 3, 4, 8], the patient was considered unsuitable for an intensive therapeutic program. Therefore, standard treatment with R-CHOP and prophylactic granulocyte-colony stimulating factor treatment were given. The patient received six R-CHOP courses with no significant toxicity, although she required RBC and platelet transfusions until the commencement of the third course, the latter administered with prophylactic intent given her extremely low platelet count. After the second R-CHOP cycle, the peripheral blood count improved significantly, and the patient achieved transfusion independence with regard to both RBC units and platelet concentrates.
At the time of completion of the planned program (six R-CHOP courses), BM revaluation by trephine biopsy showed no remnant lymphoma cells. Results of the PET scan were normal. Complete remission (CR) of DLBCL was therefore demonstrated, and two additional courses of R-CHOP were administered as consolidation therapy. Twenty-four months since the end of the patient's course of immunochemotherapy, she is well with no sign of disease, as demonstrated by recent clinical reevaluations, including a whole-body PET scan and BM trephine biopsy.
In conclusion, we describe a case of an elderly and frail patient with primary DLBCL of the BM that was discovered during work-up for pancytopenia, which was initially suspected to be caused by MDS; the patient achieved long-lasting CR that was maintained for 20 months following the completion of eight courses of R-CHOP. Primary DLBCL of the BM is a rare but distinctive form of extranodal NHL with a poor prognosis and a reported 2-year survival rate of approximately 30% and median survival time of 14.9 months [4]. Given its rarity, no standard therapy for primary DLBCL of the BM has been established. However, as R-CHOP has become the standard treatment for DLBCL in elderly patients, this case suggests that, similar to other types of DLBCL, this regimen can be a well-tolerated, frontline treatment with an efficacy to allow durable CR, even in DLBCL cases with poor prognosis such as ours.
The follow-up period for this patient was too short to draw any firm conclusions; however, compared with the usually dismal outcome of this type of NHL, the 24-month continuous clinical response observed in this case would be considered a satisfactory therapeutic result. In this context, R-CHOP can be considered a reasonable approach in cases where advanced age, toxicity concerns, and comorbidities may detract from the pursuit of more aggressive treatments such as high-dose chemotherapy and ASCT.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download