Abstract
Background
With the approval of topical retapamulin ointment in 2011, it was officially required to conduct a post-marketing surveillance (PMS) study to obtain further data of its safety profile and effectiveness, in accordance with the requirement of the Korean Ministry of Food and Drug Safety (MFDS).
Objective
This study had prospectively designed to monitor safety and tolerability with the effectiveness of topical retapamulin in clinical practices.
Methods
Open label, multi-center, non-interventional observational study was done from May 2011 to October 2015. All subjects had bacterial skin infections of locally approved prescribing information accordingly. The study mainly focused on safety issues in the local target population (3,612 eligible subjects). And, drug effectiveness was also evaluated by physicians.
Results
The incidence of adverse events (AEs) and adverse drug reactions (ADRs) were 2.53% and 0.97%, respectively. In terms of the incidence of unexpected AEs and ADRs, 1.45% and 0.33%, and for the incidence of serious AEs, 0.28%, whereas no serious ADRs reported. And, the effectiveness of topical retapamulin rate was 96.1% (1,697 of total 1,765 subjects).
Superficial bacterial skin infections, specifically caused by Staphylococcus aureus and Streptococcus pyogenes, have been commonly managed with topical antibiotics such as fusidic acid and mupirocin in Korea. And, retapamulin has also become available in this country from July 2011, as an additional topical antibiotic agent for the short-term treatment of several types of superficial skin infections.
Retapamulin is an antibacterial agent with a novel structure and a distinct mode of action. It was classified as a member of a new chemical class of antibacterial agents for human use known as pleuromutilins, which became the first drug of the pleuromutilin class to be registered anywhere in the world for human use. A semisynthetic derivative of the compound pleuromutilin, which is isolated through fermentation from the fungus Clitopilus passeckerianus, selectively inhibits bacterial protein synthesis by interacting with the 50s subunit of the bacterial ribosome in a novel manner12.
It has been consistently said that retapamulin showed an excellent in vitro activity against Gram-positive bacteria, commonly associated with skin infections, and a low tendency of resistance in vitro, which suggests that drug resistance would not easily develop during the administration of topical retapamulin3.
The objective of this study was to explore adverse events (AEs) of topical retapamulin, including drug effectiveness, in Korean patients with superficial skin infections as per the requirement of Korean Ministry of Food and Drug Safety (MFDS). This would be the first report to publish the real-world therapeutic profile of retapamulin in Korea, especially based on the data collected from a large population more than 3,000 Korean patients.
With the approval of topical retapamulin ointment in 2011, it was officially required to conduct a post-marketing surveillance (PMS) study to obtain further data of its safety profile and effectiveness, in accordance with the requirement of MFDS as a condition of market authorization in Korea.
This PMS study for a topical retapamulin (protocol 115579, funded by GlaxoSmithKline Korea and registered on ClinicalTrials.gov as NCT01445600) was conducted as an open label, multicenter, and non-interventional observational research. And, in compliance with the requirements of the Korean MFDS, a minimum of 3,000 subjects should be enrolled for this PMS study. Study information was gathered as a part of routine clinical monitoring of subjects and collected in a standardized electronic case report form. The institutional review boards approved the study protocol.
All subjects must satisfy the following criteria at study entry. 1) Subjects topically administered with a topical retapamulin for a short-term treatment of following superficial skin infections: impetigo and infected small lacerations, abrasions or sutured wounds. 2) Subjects who the investigator believes that they can manage as per the requirements of the protocol and the administration regimen. 3) Subjects administered with a topical retapamulin following the locally approved prescribing information accordingly.
1) Subjects with a known or suspected hypersensitivity to retapamulin or any component of the ointment. 2) Infants under nine months of age.
An AE, coded based on World Health Organization Adverse Reactions Terminology 092 (WHO-ART 092), was defined as any untoward medical occurrence in a subject temporally associated with the use of a topical retapamulin, regardless of its correlation with the medicinal product4. Serious AE (SAE) is any untoward following medical occurrence at any dose; 1) death, 2) life-threatening, 3) inpatient hospitalization or prolongation of existing hospitalization, 4) persistent or significant disability/incapacity, or 5) is a congenital anomaly/birth defect.
An adverse drug reaction (ADR) was defined as all noxious and unintended responses related to a topical retapamulin. Physicians classified the relatedness into 6 categories; “certain,” “probable/likely,” “possible,” “unlikely,” “conditional/unclassified,” and “unassessable/unclassifiable.” If the causality between a topical retapamulin and an AE is considered “certain,” “probable/likely,” “possible,” “conditional/unclassified,” or “unassessable/unclassifiable,” this AE was classified as an ADR.
The primary endpoint was occurrence rates of AEs after the administration of topical retapamulin. And a secondary endpoint specifically included occurrence rates of either unexpected or SAEs during the treatment using a topical retapamulin. And effectiveness of a topical retapamulin was assessed by reviewing clinical signs and symptoms at the end of therapy evaluation, which was also regarded as an additional secondary endpoint.
The clinical outcome and treatment response was assigned for each subject. The effectiveness of a topical retapamulin was evaluated as follows: 1) total absence of the treated lesions, 2) the treated lesions have become dry without crusts compared to baseline, 3) improvement (defined as a decline in the size of the affected area, number of lesions or both) such that no further antimicrobial therapy is necessary.
Statistical analysis for this study was designed to descriptively interpret the data, which quantitatively describe or summarize features of a collection of information, not to test any statistical hypothesis.
A total of 3,612 subjects were initially enrolled into this PMS study. But, finally 3,594 subjects were selected for safety evaluation, except information of the other 18 subjects that were found to be improperly handled. The evaluation of drug effectiveness was possible only for 1,765 subjects, considering the follow-up loss of the other 1,829 subjects.
Patient data was collected from 10th July 2009 to 9th July 2015. And their mean age was 40.3±22.6 years old. The sex ratio (male:female=1,792:1,802) of all these subjects was about 1:1 (49.9:50.1) (Table 1). And the age was ranged from 1 to 93 years old.
We have counted all the patient's diagnoses in a duplicate manner, and the results are as follows: 425 patients (11.8%) suffered from impetigo, and 3,207 patients (89.2%) showed infected small lacerations, abrasions, or sutured wound. The mean period of retapamulin treatment was 10.0±19.9 days.
During the study period, the incidence of AEs and ADRs was 2.53% (91/3,594 subjects, 125 events) and 0.97% (35/3,594 subjects, 53 events), respectively. And the symptom, ‘Pruritus’, ranked both the most common AE (0.33%, 12/3,594 subjects, 12 events) and the most common ADR (0.19%, 7/3,594 subjects, 7 events).
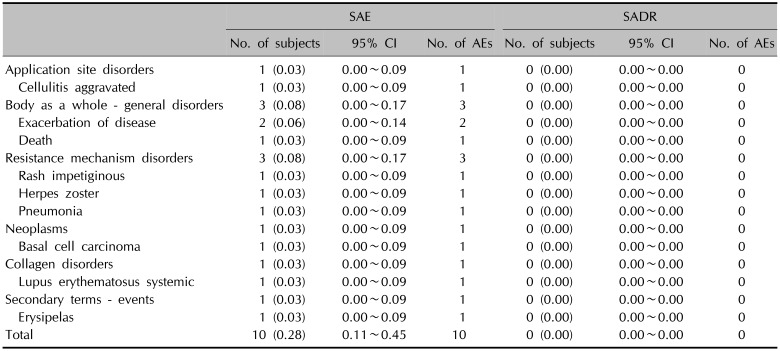
The incidence of unexpected AEs and unexpected ADRs was 1.45% (52/3,594 subjects, 68 events) and 0.33% (12/3,594 subjects, 22 events), respectively. ‘Pain’ and ‘Constipation’ (0.08%, 3/3,594 subjects, 3 events) were the most common unexpected AEs. While ‘Skin disorder,’ ‘Oedema,’ ‘Temperature changed sensation,’ and ‘Pustular rash’ (0.06%, 2/3,594 subjects, 2 events) were the most common unexpected ADRs. The incidence of SAEs was 0.28% (10/3,594 subjects, 10 events), whereas no serious ADRs (SADRs) was officially reported. The most common SAE was ‘Exacerbation of disease’ (0.06%, 2/3,594 subjects, 2 events).
A total of 125 AEs were reported by 91 subjects (incidence=2.53%). Among the patients with these AEs, skin and appendages disorders were reported as follows: 12 cases of pruritus, 5 cases of erythema, 3 cases of eczema, 2 cases of bullous eruption, and 2 cases of skin disorders. And they also showed symptoms such as papular rash, urticaria, increased sweating, maculo-papular rash, rash, verruca, acne, otitis externa, contact dermatitis, skin ulceration, and dermatitis in a duplicate manner.
Otherwise, a total of 53 ADRs were reported by 35 subjects (incidence=0.97%). And, specifically, skin and appendages disorders consisted of 7 cases of pruritus, 3 cases of erythema, 3 cases of eczema, 1 case of bullous eruption, 2 cases of skin disorder, 1 case of papular rash, 1 case of increased sweating, 1 case of otitis externa, and 1 case of skin ulceration. Other AEs and ADRs, except pruritus (12 subjects) and application site pruritus/irritation (8/9 subjects), were experienced by less than 5 subjects (Table 2).
A total of 68 unexpected AEs were collected in 52 subjects (incidence=1.45%). Specifically, 2 cases of bullous eruption and 2 cases of skin disorders were recorded as a category of skin and appendages disorders. Additionally, papular rash, urticarial, increased sweating, maculo-papular rash, rash, verruca, acne, otitis externa, skin ulceration, and dermatitis were also observed among these patients in a duplicate manner.
However, a total of 22 unexpected ADRs were recorded for 12 subjects (incidence=0.33%). And only 7 cases related to skin and appendages disorders (bullous eruption, skin disorder, papular rash, increased sweating, otitis externa, and skin ulceration) were reported for 6 subjects as ADRs. In terms of application site disorders, only 1 case was reported for 1 subject (Table 3).
All kinds of SAEs were collected regardless of causal relationship between SAEs and a topical retapamulin. And, these SAEs were reported immediately after the recognition as per the guideline of Korea Institute of Drug Safety & Risk Management. During the study, total 10 cases of SAEs were collected from 10 subjects (0.28%). However, none of them was officially reported as an SADR during this PMS activity (Table 4).
The effectiveness of retapamulin was assessed by comparing pre- and post-treatment states as a clinical evidence of the symptom improvement. ‘Clinical improvement’ was regarded as effective, and in terms of ‘Clinical failure,’ ineffective. The effectiveness rate was 96.1% (1,697 of total 1,765 subjects).
Recently new drugs from natural products and their semi-synthetic derivatives are expected to play an important role as an alternative to other antibacterial agents that have been used for the treatment of skin and skin structure infections (SSSIs)5. Pleuromutilins were first discovered in 1950s and have been usually applied per os in veterinary medicine since 1979 (tiamulin, valnemulin) in some European countries. The structural nature of these compounds led to the beginning for the development of this molecular class as an important antimicrobial drug6. And, retapamulin was the first pleuromutilin that was approved for the human body (2007, Europe; 2008, USA)7.
Retapamulin is a semi-synthetic pleuromutilin derivative as an antimicrobial agent against common Gram-positive pathogens in human associated with SSSIs3789. Based on this specific characteristics, retapamulin can be an adequate topical agent for the treatment of uncomplicated SSSIs610. The U.S. Food and Drug Administration approved the use of retapamulin in 2007 for the topical treatment of impetigo. The European Medicines Agency has also approved retapamulin for more expanded indications, including impetigo as well as infected small lacerations, abrasions, or sutured wounds.
It is the brief explanation of mechanism of action which retapamulin shows a bacteriostatic action against S. aureus and S. pyogenes in the human body. Retapamulin, a semi synthetic pleuromutilin antibiotic agent, inhibits protein synthesis by the interaction with 50S prokaryotic ribosomal unit. This site of retapamulin interaction is quite different from those of other antibiotics11. Pleuromutilins do not bind to eukaryotic ribosomes and do not also inhibit mammalian protein synthesis712.
Uncomplicated SSSIs (uSSSIs), such as secondarily infected traumatic lesions (SITLs) and impetigo, are common clinical conditions frequently caused by S. aureus and S. pyogenes131415. In Korea the approved-indications of retapamulin have covered uSSSIs as follows: primary impetigo, SITLs (small lacerations, abrasions, sutured wounds), and secondarily infected dermatoses (infected psoriasis, infected atopic dermatitis, and infected contact dermatitis). The recommended dose is a thin layer of ointment, twice a day for five days. Even though systemic exposure following application into intact skin is generally very low, detectable concentrations were observed in 69% of babies aged 2 to 9 months16. Retapamulin is, therefore, contraindicated in babies under nine months. As this drug is metabolized by cytochrome P450 (CYP) 3A4, inhibitors of this enzyme such as ketoconazole may increase retapamulin exposure in children under two years. The efficacy of retapamulin 1% ointment in patients aged nine months and older has been studied in several phase III trials117181920. Furthermore, retapamulin was usually recommended not to be used to treat abscesses or cellulitis and not be applied to mucosal membranes or eyes5.
Topical antibiotics are usually preferred over oral agents for the treatment of suitable uSSSIs because of their lower potential for systemic adverse effects and avoidance of resistance selection in the gut flora21. However, when prescribing antibiotics for skin infections, geographical variations in antibiotic susceptibility should be taken into account. The understand of local and regional variability in antimicrobial resistance rates is vitally important to determine appropriate and effective empiric treatment options.
This understanding should be also applied to topical antimicrobial agents, which have been extensively used for both therapeutic and decontaminating purposes22. Currently the most commonly prescribed and/or used topical antibiotics in Korea are fusidic acid and mupirocin. But, staphylococci are often resistant to both of topical agents. It was reported that the resistance rate of S. aureus to mupirocin is currently 5% to 25.3%. Most of all, high-level mupirocin resistant strains, which cannot be controlled by mupirocin, have been increasing in prevalence. These strains are more clinically important23. As for fusidic acid, there was a report that a resistance rate to fusidic acid of 23.9% in 2006. Unfortunately, the recent study, published in 2016, has shown that the resistance has increased to a rate of 44.0%24.
Now it has become to be unavoidable to identify effective alternatives to these current topical antibiotics, to which microbial pathogens have continued to evolve resistances. Hence, the regulatory approvals of antimicrobial agents over the past several years, such as daptomycin, retapamulin and fidaxomicin, can represent the beginning of a new modern antibiotic period5.
In that regard, Korean investigators suggested in their recently-published studies that retapamulin was highly active in vitro against Korean clinical isolates of high-level mupirocin-and methicillin-resistant S. aureus (MRSA) with different genetic backgrounds24. And there was another similar report about retapamulin and its antimicrobial susceptibility patterns. Researchers in USA also proposed that although the use of mupirocin has been the standard therapy for decolonization practices as of now, the activity of retapamulin could warrant its consideration as an alternative therapy in MRSA decolonization regimens25.
The results from this PMS study for topical retapamulin in Korea, obtained during the re-examination period of retapamulin, did not reveal any crucial issues that could affect the drug safety and efficacy. Application site reactions were one of the most frequently reported AEs with retapamulin. In addition, irritation, pruritus, paraesthesia and pain were also observed during study period. In most the trials, including this large retapamulin PMS study, these AEs were actually reported by less than 2% to 3% of total patients1718192026. In total, AEs and ADRs were recorded for 2.53% and 0.97% of patients, respectively. And the most frequently reported AE and ADR were ‘Pruritus’. Unexpected AEs and ADRs were also recorded for less than 2% of patients. And, only 0.28% of total patients reported SAEs and no patients experienced SADR during this study period. Based on this safety result, retapamulin can be regarded as being well-tolerated for superficial skin infection (representatively, impetigo or infected small lacerations, abrasions, or sutured wounds) in real life practices. Additionally, in this PMS study, we could roughly see that the effectiveness rate of retapamulin treatment in Korea was very high, 96.1% (1,697 of total 1,765 subjects).
However, there are some limitations in this study in terms of assessment of drug safety and effectiveness in a real practice. Firstly, it should be considered that we could reliably detect only a small fraction of the range of possible drug-related events without controlled groups. Secondly, 1,829 subjects out of 3,594 in the safety population could not be assessed for effectiveness due to the follow-up loss. The incomplete result data due to those subjects, who were not assessed for effectiveness, may have induced withdrawal bias.
Topical retapamulin can be considered safe and effective in its approved treatment indications of Korea. And, for more accurate understanding of retapamulin in this country, it would be helpful to continue a close monitoring for an AE incidence and its relationship with this topical agent in the future.
Notes
CONFLICTS OF INTEREST: Woosung Hong and Yil-Seob Lee are employees of GlaxoSmithKline and own company shares, which had no influence on the present work. The other authors have nothing to disclose.
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
References
1. Champney WS, Rodgers WK. Retapamulin inhibition of translation and 50S ribosomal subunit formation in staphylococcus aureus cells. Antimicrob Agents Chemother. 2007; 51:3385–3387. PMID: 17562806.
2. Wolf R, Davidovici BB, Parish JL, Parish LC. Emergency dermatology. Cambridge, USA: Cambridge University Press;2011. p. 376.
3. Jones RN, Fritsche TR, Sader HS, Ross JE. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob Agents Chemother. 2006; 50:2583–2586. PMID: 16801451.

4. Korea Institute of Drug Safety & Risk Management. WHO-ART 092 [Internet]. Anyang: Korea Institute of Drug Safety & Risk Management;2015. 7. cited 2018 Jun 8. Available from: https://www.drugsafe.or.kr/iwt/ds/ko/bbs/EgovBbs.do;jsessionid=JcVbUVsrD2anWzye6BgkPqwiw4cktqW0oiFbNuq2WQaDVhqlgoXgLidzeTEGPuTz.webint_2_servlet_engine1?bbsId=BBSMSTR_000000000024&nttId=1446&pageIndex=2&searchCnd=&searchWrd=.
5. Kirst HA. Developing new antibacterials through natural product research. Expert Opin Drug Discov. 2013; 8:479–493. PMID: 23480029.

6. Yang LP, Keam SJ. Retapamulin: a review of its use in the management of impetigo and other uncomplicated superficial skin infections. Drugs. 2008; 68:855–873. PMID: 18416589.
7. Tang YZ, Liu YH, Chen JX. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini Rev Med Chem. 2012; 12:53–61. PMID: 22070694.
8. Scangarella-Oman NE, Shawar RM, Bouchillon S, Hoban D. Microbiological profile of a new topical antibacterial: retapamulin ointment 1%. Expert Rev Anti Infect Ther. 2009; 7:269–279. PMID: 19344241.

9. Jacobs MR. Retapamulin: a semisynthetic pleuromutilin compound for topical treatment of skin infections in adults and children. Future Microbial. 2007; 2:591–600.

10. Parish LC, Parish JL. Retapamulin: a new topical antibiotic for the treatment of uncomplicated skin infections. Drugs Today (Barc). 2008; 44:91–102.

11. Physician's desk reference 2009. 63rd ed. Montvale: Thomson PDR;2008.
12. Novak R. Are pleuromutilin antibiotics finally fit for human use? Ann N Y Acad Sci. 2011; 1241:71–81. PMID: 22191527.

13. Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005; 41:1373–1406. PMID: 16231249.

14. Silverberg N, Block S. Uncomplicated skin and skin structure infections in children: diagnosis and current treatment options in the United States. Clin Pediatr (Phila). 2008; 47:211–219. PMID: 18354031.

15. Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, et al. New insights into meticillin-resistant staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents. 2012; 39:96–104. PMID: 22196394.

16. Food and Drug Administration (FDA). Prescribing information: ALTABAX® (retapamulin ointment), 1%. Silver Spring: FDA;2012.
17. Koning S, van der Wouden JC, Chosidow O, Twynholm M, Singh KP, Scangarella N, et al. Efficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trial. Br J Dermatol. 2008; 158:1077–1082. PMID: 18341664.

18. Oranje AP, Chosidow O, Sacchidanand S, Todd G, Singh K, Scangarella N, et al. Topical retapamulin ointment, 1%, versus sodium fusidate ointment, 2%, for impetigo: a randomized, observer-blinded, noninferiority study. Dermatology. 2007; 215:331–340. PMID: 17911992.

19. Tomayko JF, Li G, Breton JJ, Scangarella-Oman N, Dalessandro M, Martin M. The safety and efficacy of topical retapamulin ointment versus placebo ointment in the treatment of secondarily infected traumatic lesions: a randomized, double-blind superiority study. Adv Skin Wound Care. 2013; 26:113–121. PMID: 23426412.
20. Parish LC, Jorizzo JL, Breton JJ, Hirman JW, Scangarella NE, Shawar RM, et al. Topical retapamulin ointment (1%, wt/wt) twice daily for 5 days versus oral cephalexin twice daily for 10 days in the treatment of secondarily infected dermatitis: results of a randomized controlled trial. J Am Acad Dermatol. 2006; 55:1003–1013. PMID: 17097398.

21. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010; 74:417–433. PMID: 20805405.

22. Biedenbach DJ, Bouchillon SK, Johnson SA, Hoban DJ, Hackel M. Susceptibility of staphylococcus aureus to topical agents in the United States: a sentinel study. Clin Ther. 2014; 36:953–960. PMID: 24835558.

23. Park SY, Kim SM, Park SD. The prevalence, genotype and antimicrobial susceptibility of high- and low-level mupirocin resistant methicillin-resistant staphylococcus aureus. Ann Dermatol. 2012; 24:32–38. PMID: 22363153.
24. Park SH, Kim JK, Park K. In vitro antimicrobial activities of fusidic acid and retapamulin against mupirocin- and methicillin-resistant staphylococcus aureus. Ann Dermatol. 2015; 27:551–556. PMID: 26512169.
25. Harrington AT, Black JA, Clarridge JE 3rd. In vitro activity of retapamulin and antimicrobial susceptibility patterns in a longitudinal collection of methicillin-resistant staphylococcus aureus isolates from a veterans affairs medical center. Antimicrob Agents Chemother. 2015; 60:1298–1303. PMID: 26666950.
26. Free A, Roth E, Dalessandro M, Hiram J, Scangarella N, Shawar R, et al. Retapamulin ointment twice daily for 5 days vs oral cephalexin twice daily for 10 days for empiric treatment of secondarily infected traumatic lesions of the skin. Skinmed. 2006; 5:224–232. PMID: 16957433.

Table 1
Demographics and baseline characteristics
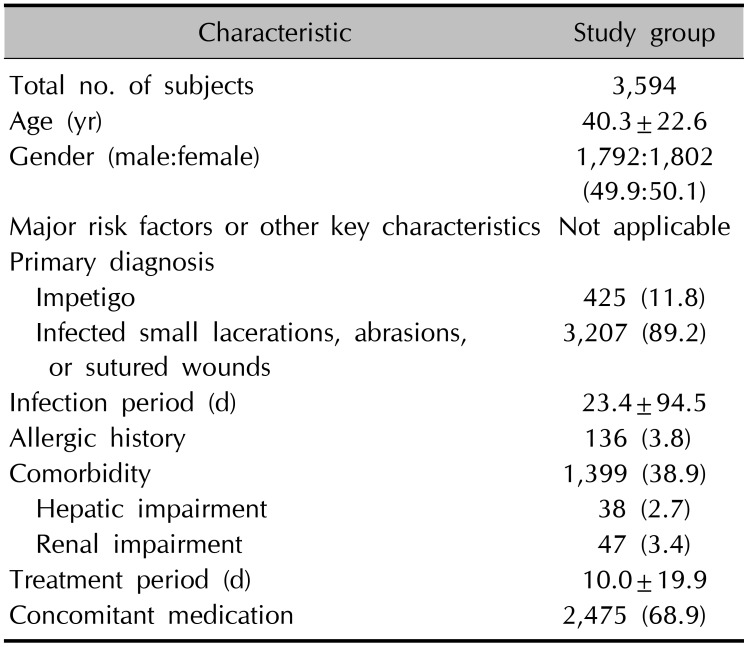
Table 2
Incidence of AEs and ADR (n=3,594)
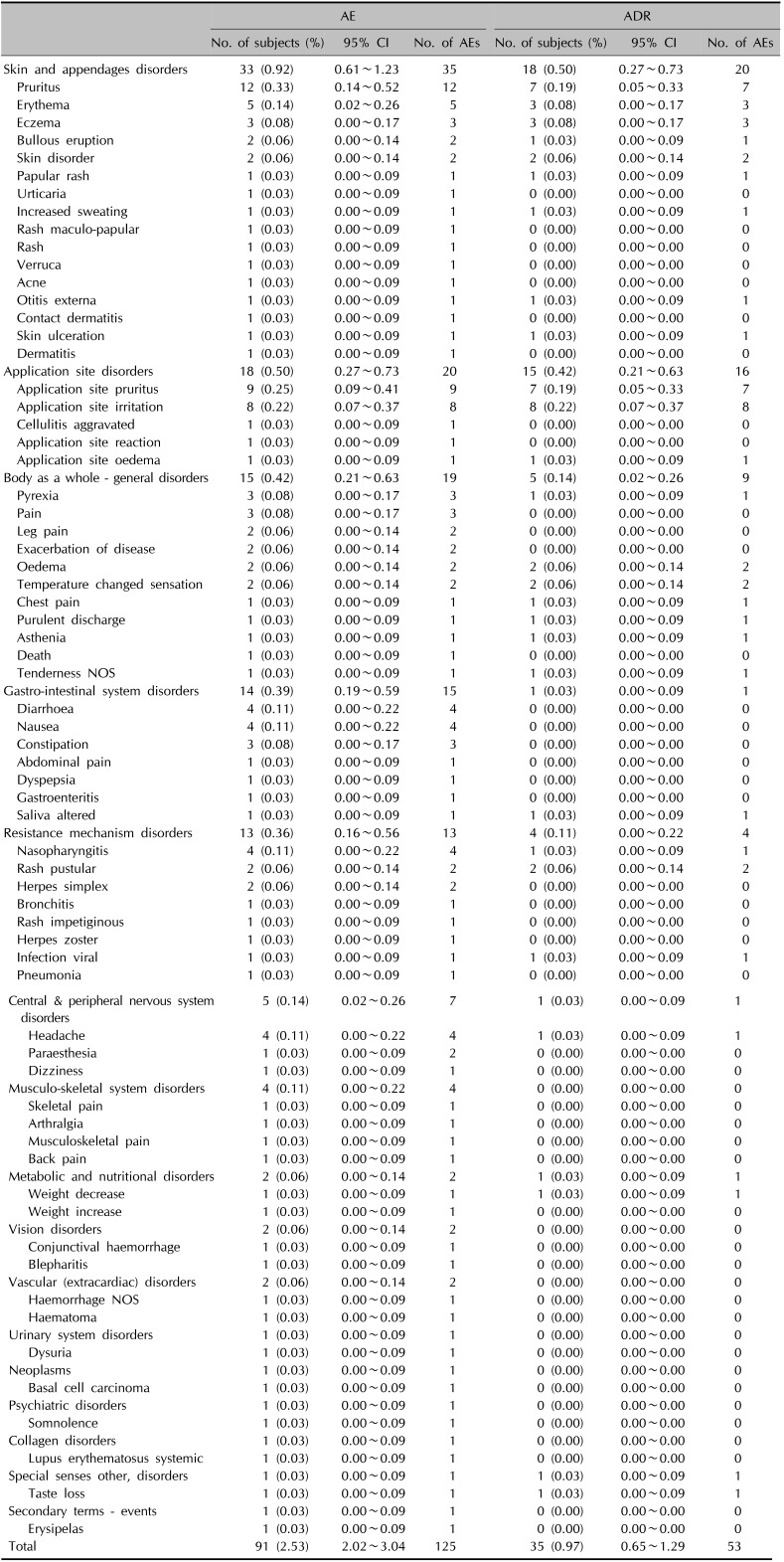
Table 3
Incidence of unexpected AEs and unexpected ADR (n=3,594)
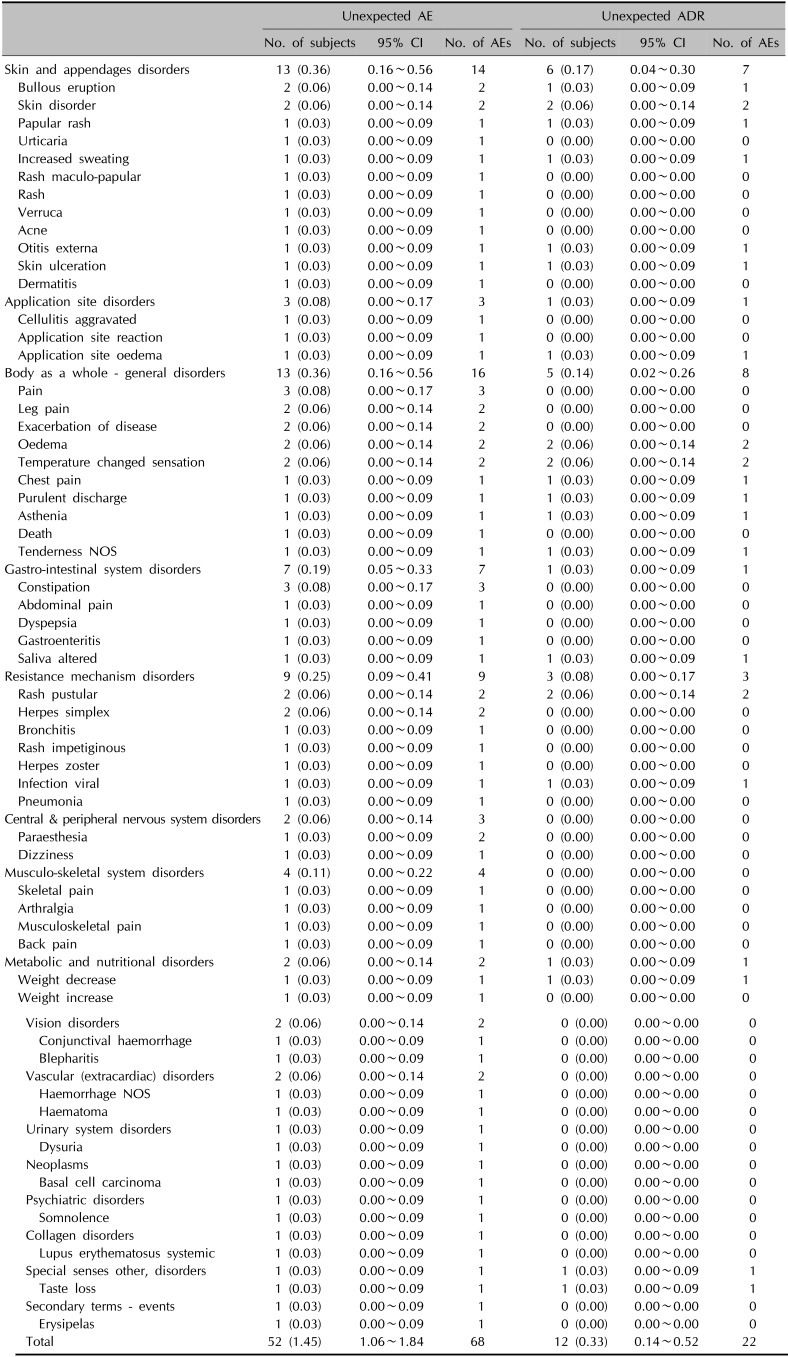
Table 4
Incidence of SAEs and SADR (n=3,594)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download