1. Uehara M, Kimura C. Descendant family history of atopic dermatitis. Acta Derm Venereol. 1993; 73:62–63. PMID:
8095756.
2. Kryukov GV, Pennacchio LA, Sunyaev SR. Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am J Hum Genet. 2007; 80:727–739. PMID:
17357078.

3. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015; 66(Suppl 1):8–16. PMID:
25925336.

4. Li K, Oh WJ, Park KY, Kim KH, Seo SJ. FLG mutations in the East Asian atopic dermatitis patients: genetic and clinical implication. Exp Dermatol. 2016; 25:816–818. PMID:
27120251.
5. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011; 365:1315–1327. PMID:
21991953.

6. Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet. 2014; 59:5–15. PMID:
24196381.

7. Pabinger S, Dander A, Fischer M, Snajder R, Sperk M, Efremova M, et al. A survey of tools for variant analysis of next-generation genome sequencing data. Brief Bioinform. 2014; 15:256–278. PMID:
23341494.

8. Thomas PD, Kejariwal A. Coding single-nucleotide polymorphisms associated with complex vs. Mendelian disease: evolutionary evidence for differences in molecular effects. Proc Natl Acad Sci U S A. 2004; 101:15398–15403. PMID:
15492219.

9. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25:1754–1760. PMID:
19451168.

10. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011; 43:491–498. PMID:
21478889.

11. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013; 31:213–219. PMID:
23396013.

12. Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013; 68:498–506. PMID:
23452057.

13. Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016; 12:52. PMID:
27777593.

14. Kazma R, Bailey JN. Population-based and family-based designs to analyze rare variants in complex diseases. Genet Epidemiol. 2011; 35(Suppl 1):S41–S47. PMID:
22128057.

15. Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2012; 13:135–145. PMID:
22251874.

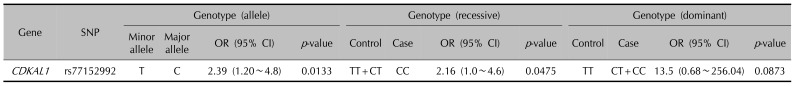
16. Quaranta M, Burden AD, Griffiths CE, Worthington J, Barker JN, Trembath RC, et al. Differential contribution of CDKAL1 variants to psoriasis, Crohn's disease and type II diabetes. Genes Immun. 2009; 10:654–658. PMID:
19587699.

17. Coto-Segura P, Batalla A, González-Fernández D, Gómez J, Santos-Juanes J, Queiro R, et al. CDKAL1 gene variants affect the anti-TNF response among Psoriasis patients. Int Immunopharmacol. 2015; 29:947–949. PMID:
26563541.

18. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015; 78:89–94. PMID:
25771165.

19. Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005; 15:1034–1050. PMID:
16024819.

20. Ladinsky HT, Elizalde A, Schickler R, Dees PB, Crenshaw ML, Sleasman JW. Hypereosinophilic syndrome and hemimelia in a patient with chromosome 6p22.3 deletion. Pediatr Allergy Immunol. 2014; 25:500–503. PMID:
24628666.

21. Jenerowicz D, Czarnecka-Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol Alp Pannonica Adriat. 2007; 16:47–52. PMID:
17992457.
22. Ott H, Stanzel S, Ocklenburg C, Merk HF, Baron JM, Lehmann S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta Derm Venereol. 2009; 89:257–261. PMID:
19479121.

23. Song GG, Lee YH. Pathway analysis of genome-wide association study on asthma. Hum Immunol. 2013; 74:256–260. PMID:
23200760.

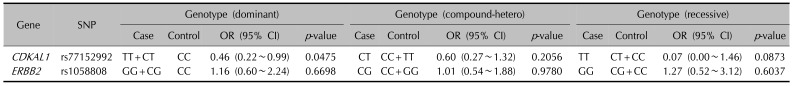
24. Sääf A, Pivarcsi A, Winge MC, Wahlgren CF, Homey B, Nordenskjöld M, et al. Characterization of EGFR and ErbB2 expression in atopic dermatitis patients. Arch Dermatol Res. 2012; 304:773–780. PMID:
22552355.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download