This article has been corrected. See "Erratum: Folliculotropic Mycosis Fungoides in 20 Korean Cases: Clinical and Histopathologic Features and Response to Ultraviolet A-1 and/or Photodynamic Therapy" in Volume 30 on page 510.
Abstract
Background
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) that is characterized clinically by variable types of skin eruptions, including plaques, acneiform lesions, and alopecic patches. Histopathologically, FMF is characterized by folliculotropic infiltrates.
Objective
This study was conducted to scrutinize the clinical and histopathologic features of FMF in Koreans and the responses to phototherapy.
Methods
Twenty Koreans diagnosed with MF who had histopathologic evidence of folliculotropism were enrolled.
Results
Eighteen patients had head-and-neck-region infiltration, while five had solitary lesion. In all patients, the atypical lymphocytic infiltrate had a perifollicular distribution. Twelve patients were treated with ultraviolet A (UVA)-1. Eleven of these 12 patients with early-stage FMF experienced >80% improvement (8: complete remission; 3: partial remission). Four patients, including 2 who relapsed after UVA-1, were treated with photodynamic therapy (PDT), reaching complete remission after PDT.
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL)12. The folliculotropic form of MF differs clinically and histopathologically from classic MF. Therefore, it is important to better understand the characteristics of this variant form3. Folliculotropic mycosis fungoides (FMF) is a common subtype of MF4, which is characterized by various types of skin eruptions, including infiltrative plaques, acneiform lesions, cysts, and alopecic patches. Histopathologic findings showed folliculotropic infiltrates.
Ultraviolet A-1 (UVA-1) phototherapy was first described in 1978, and has since become a valuable treatment for sclerotic and T-cell mediated diseases. Compared to UVB, UVA-1 penetrates into the deeper layers of reticular dermis5. Previously, some have investigated the therapeutic effects of UVA-1 on MF. However, only a few studies have addressed UVA-1 phototherapy in FMF. In this study, we scrutinized the clinical, pathological, and immunohistochemical features of FMF in 20 Korean patients and evaluated patients' responses to phototherapy, especially with regard to UVA-1 and photodynamic therapy (PDT).
This study included 20 patients with histopathologic evidence of folliculotropism who were diagnosed with MF at the Department of Dermatology, Kosin University Gospel Hospital, Busan, Korea. This study was approved by the Institutional Review Board of the Kosin University Gospel Hospial (IRB no. KUGH 2017-01-001).
The following data were collected: patient age, gender, disease duration, distribution of skin lesion, symptoms, TNM stage, treatment responses, and follow up.
Biopsies were conducted on follicular (comedo-like, cystic, and acneiform) lesions. We observed the histopathologic features of typical MF and evidence of folliculotropism. Paraffin-embedded tissue sections were stained using monoclonal antibodies against CD4 and CD8.
UVA-1 phototherapy was delivered using SELLAMED 3000 (Sellas Medizinische Gerate GmbH, Gevelsberg, Germany). Patients were treated with medium-dose (65 J/cm2) or high-dose (100 J/cm2) UVA-1 phototherapy. The main wavelengths were emitted from 340 nm to 400 nm. The irradiation intensity of SELLAMED 3000 was 70 mW/cm2 at a distance of 30 cm. The frequency of therapy ranged from 3 to 5 times weekly. For PDT, a 16.8% methyl aminolevulinate (MAL) cream (Metvix® cream; Galderma, Paris, France) was applied topically to the lesion in a 1-mm-thickness with a 5-mm border extending to the normal skin. The lesion was then covered with an occlusive, light-shielding dressing. After 3 hours, the dressings were removed, and the cream was washed off with a 0.9% saline solution. The lesions were irradiated with red light from a light-emitting diode (Aktilite CL128; PhotoCure ASA, Oslo, Norway) at a mean wavelength of 630 nm, a total light dosage of 37.5 J/cm2, and an irradiation intensity of 75 mW/cm2 at skin level for 8 minutes 20 seconds. The patients received MAL-PDT sessions once per month. In order to evaluate therapeutic efficacy, clinical photographs were taken at every visit with the same digital camera (α350; Sony, Tokyo, Japan), in the same posture, and under controlled lighting conditions. Two independent dermatologists assessed patients' responses to phototherapy based on these photographs. The clinical response was defined as complete improvement (≥95% clinical improvement); partial improvement (≥50% clinical improvement); or no response (<50% clinical improvement). All the patients were followed every 1 to 2 months to detect any recurrence after the treatment was completed.
Twenty (5.5%) of the 366 MF patients had FMF. Twelve (60.0%) of these 20 patients were male, and eight (40.0%) were female. The patients' ages ranged from 32 years to 68 years, with a mean of 47.4 years. The disease duration ranged from 3 months to 2 years, with a mean of 1.9 years (Table 1). Eighteen of 20 patients experienced itching, among whom 13 had intense pruritus. The remaining 2 had no subjective symptoms. The most common involved site was the head and neck (90.0%). Six (30.0%) patients had lesions on the face, while five (25.0%) had lesions on the extremities. Five patients (25.0%) had solitary lesions. Skin lesions presented as follicular papules in 12 patients (60.0%), folliculocentric lesions in 2 patients (10.0%), patch/plaques in 19 patients (95.0%), and erythroderma in 3 patients (15.0%) (Table 2). Interestingly, agminated follicular lesions were identified in 3 patients. In addition, patients who showed erythroderma previously were found to have MF presenting as Ofuji's papuloerythroderma and folliculotropic Sézary syndrome (FSS). In this study, alopecia was observed in a patient (#15), which manifested as eyebrow loss. Four patients were previously diagnosed with conventional MF; among them, patient #10 had a concurrent ichthyosiform lesion. According to the TNM classification, 15 patients (75.0%) had stage IA, 3 (15.0%) had stage IB, and 2 (10.0%) had stage IVA disease (Fig. 1, 2, 3, 4, Table 1).
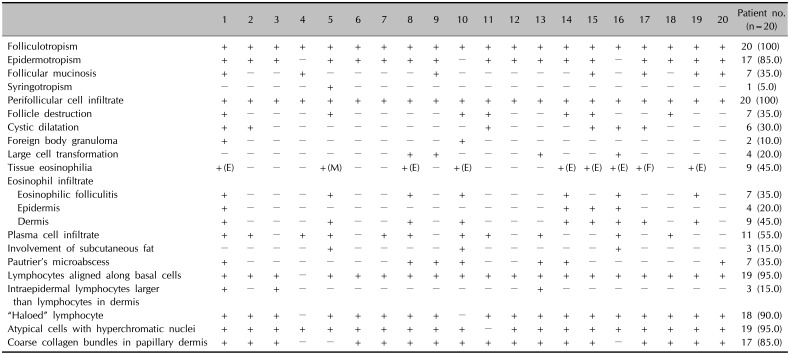
The atypical lymphocytic infiltrates had a perifollicular distribution in all patients (100%). Epidermotropism was observed in 17 patients (85.0%). Follicular mucinosis was observed in 7 patients (35.0%). Syringotropism was observed in 1 patient (5.0%). All 20 patients had a perifollicular cell infiltrate (100%). There was follicular destruction in 7 patients (35.0%). Cystic dilatation was observed in 6 patients (30.0%). Foreign body granuloma was observed in 2 patients (10.0%). Large cell transformation (LCT) was observed in 4 patients (20.0%). In addition, tissue eosinophilia was observed in 9 patients (45.0%). Seven of these 9 patients had an eosinophil infiltrate with eosinophilic folliculitis (35.0%). Eosinophil infiltrate in the epidermis was observed in 4 patients (20.0%). Eosinophil infiltrate in the dermis was observed in 9 patients (45.0%). Plasma cell infiltrate was observed in 11 patients (55.0%). The involvement of subcutaneous fat was observed in 3 patients (15.0%).
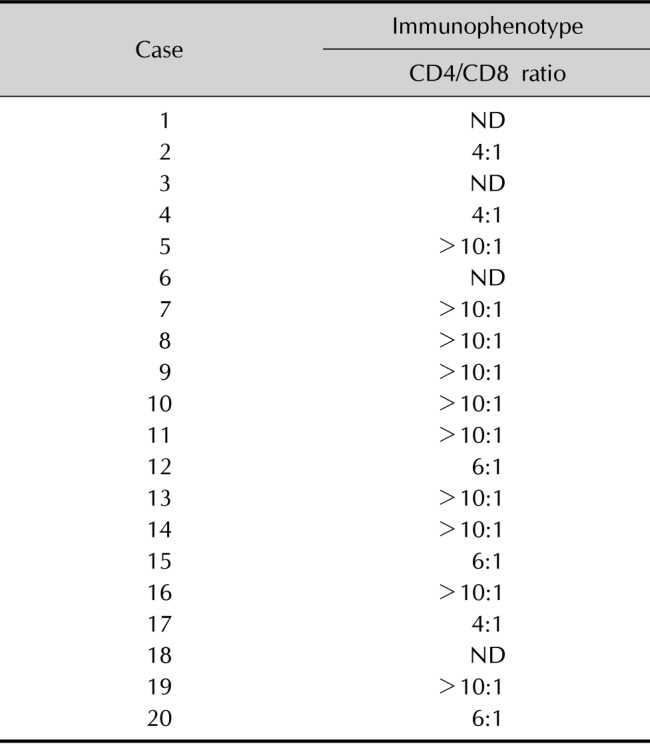
In most patients, the atypical lymphocytes expressed both CD4 and CD8 antigens. In addition, most patients had a predominance of CD4-positive T cells, compared to CD8-positive T cells. The CD4/CD8 ratio was >10 in 10 patients (Table 4).
Phototherapy was conducted in 16 of the 20 patients. Specifically, UVA-1 phototherapy, psoralen plus UVA (PUVA), and MAL-PDT were performed in 12, 2, and 2 patients, respectively. Among the 12 patients who were treated with UVA-1, 9 achieved complete remission (CR), while 3 showed partial remission (PR). Among those who had CR, 5 experienced a relapse. Two of these 5 patients were retreated with UVA-1, and then one reached CR, while the other is still undergoing treatment (given intermittent deterioration, as well as some improvement). Two patients who initially experienced relapse received PDT, and then both achieved CR. One patient (#8) responded to UVA-1 phototherapy initially, but later recurred as MF presenting as Ofuji's papuloerythroderma, and eventually died. Among all the patients, 2 were treated with PUVA, and one reached CR. Another patient reached PR, but primary cutaneous anaplastic large-cell lymphoma (PCALCL) developed 10 months later. Two patients were treated with PDT from the beginning, and both achieved CR. One patient (patient #9) was confirmed to have FSS and died despite chemotherapy. Of the 20 patients with FMF, 2 died of lymphoma during a mean 14.2 months of follow-up (Table 1).
The clinical presentation of FMF often differs from the patches and plaques in conventional MF and could not make the clinical impression of MF. Ultimately, this discrepancy can delay the diagnosis of FMF6. The most noticeable difference between FMF and conventional MF is the distribution of lesion. Conventional MF develops in a bathing suit distribution, with sparing of the head-and-neck region. In contrast, FMF has a predilection for the head-and-neck region789. In agreement with previous studies, most patients (90.0%) in this study had head-and-neck involvement. The extent of the lesions was also quite variable4. FMF often presented as a solitary lesion, which is called unilesional FMF. In a small number of patients, but more frequently, there were more extensive lesions involving >10% of the skin surface. In contrast to a previous study in which unilesional FMF did not have predilection for the head-and-neck-region10, all 5 cases of unilesional FMF in this study involved the face. Unilesional FMF has an excellent prognosis, in sharp contrast to extensive FMF, which has a poor prognosis in many previous reports. None of the unilesional FMF patients described in the literature had multifocal cutaneous or internal spread10. Among 5 patients with unilesional FMF in this study, one was treated with UVA-1 for a lesion that was solitary at initial diagnosis and achieved CR. However, the patient then experienced relapse 5 months later, with the development of multiple lesions. However, all 5 patients with unilesional FMF who were treated with phototherapy (4 patients with UVA-1, 1 patient with MAL-PDT) eventually achieved CR, including the patient who relapsed. Patients with FMF usually present with follicular erythematous papules and plaques, alopecia, and folliculocentric lesions. Follicular papules were observed in 12 patients (60.0%), folliculocentric lesions in 2 (10.0%), and patches/plaques in 19 (95.0%). In 3 patients (#5, #16, #19), there were agminated lesions characterized by prominent follicular distribution of the lesions without the interfollicular epidermis involvment11. Agminated lesions can be easily overlooked; therefore, recognizing such skin lesion as a possible clinical manifestation of FMF is important in the diagnosis of FMF. Previous studies have reported FMF cases were accompanied by classical MF or ichthyosiform MF12. We also observed FMF patients with concurrent classical MF or ichthyosiform MF. In very rare cases, FMF presents with diffuse erythroderma. In this study, diffuse erythroderma occurred in 3 patients. In 2 of these patients, the erythroderma progressed to stage IVB, and they died of FMF despite the continuous treatment. Therefore, the presence of erythroderma appears to be a poor prognostic factor. Two of these patients had the ‘deck-chair sign,’ in which there are pruritic erythematous papules and extensive erythema sparing all skin folds. This is characteristic clinical feature of Ofuji's papuloerythroderma, which most likely represents a variant of MF with a similar clinical presentation, protracted course, and high peripheral eosinophilia1113. In addition, one of 3 patients with erythroderma was diagnosed with FSS characterized by both folliculotropism and leukemic involvement. These two manifestations are aggressive variants of MF, which have a more fatal clinical course than classical MF and tend to be resistant to skin-directed therapies14.
In patients with CTCL, blood eosinophilia and activation of tissue eosinophilia are poor prognostic factors15. In one previous study, the prominent eosinophilic infiltration was a reasonably specific marker of associated pruritus6. In this study, 9 patients had findings of tissue eosinophilia. Four of these patients had a relapse and one of them died of FMF. One of 9 patients developed PCALCL 10 months after treatment. Among the 4 patients who had been diagnosed with conventional MF in this study, tissue eosinophilia was observed in 3 patients. In addition, the 9 patients with tissue eosinophilia all complained of intense pruritus. Patients with MF and Sézary syndrome in advanced stage IIB-IV disease have a high incidence of LCT. In previous studies on large cohorts of MF patients, FMF was associated with poorer survival16. In the patients with transformation of skin lesions alone, FMF was a strong and independent predictor of reduced survival17. In this study, LCT occurred in 4 patients (20.0%), 2 of whom died several years after LCT was diagnosed. The other 2 patients are still receiving treatment. Unfortunately, however, there has not been a satisfactory effect to date. In prospective cohort study of 203 patients with FMF, van Santen et al.18 reported that clinical stage, age over 60 years old, LCT, and extensive secondary bacterial infection at the time of first presentation were independent prognostic factors for disease progression and/or poor survival. In this study, although the number of patients is small, diffuse erythroderma, LCT, and tissue eosinophilia may be associated with poor prognosis and a solitary lesion was shown to be good prognostic factor.
Other studies have found that FMF is less responsive to treatment than classic MF6. In FMF, lymphocytic infiltrates penetrate deeper into the hair follicles, which limit the response to superficial treatments such as topical corticosteroids and UVB phototherapy819. There are few reports in the literature regarding use of UVA-1 phototherapy in the treatment of FMF. UVA-1 has many advantages in the treatment of FMF. For instance, it penetrates deeper than PUVA and narrowband UVB2021. Due to such skin penetration properties, hair follicle cells are also vulnerable to UVA-122. In addition, UVA-1 induces an immediate apoptotic cell death in human T lymphocytes20. Yamauchi et al.23 found that malignant T cells are more sensitive than normal cells in UVA-1 radiation-induced apoptosis. UVA-1 also has a sensitive effect on neoplastic cells by increasing the production of TNF-α. Because of these features, UVA-1 shows more favorable and faster therapeutic effectiveness on papular or folliculocenteric lesions with cell infiltration at the relatively deeper sites which is characteristic presentation of FMF. Fortunately, UVA-1 does not require psoralen, thereby lowering the risk of phototoxic reactions, compared to that with photochemotherapy24. Given these properties, UVA-1 can also be used as a maintenance therapy. In this study, 12 patients were treated with UVA-1 phototherapy. The lesions in 11 of 12 patients with early-stage MF improved by more than 80% with this modality (8: CR; 3: PR).
The treatment mechanism of PDT on MF has not been clearly established. One hypothesis suggests that PDT generates reactive oxygen species and directly destroys malignant lymphocytes through a PDT-induced inflammatory reaction25. However, there are few studies that have shown the therapeutic effects of PDT in FMF patients. In one previous study, 8 lesions on the face and neck were treated with PDT using 16.8% MAL cream. Seven of these lesions (88%) achieved CR; therefore, the group reported that MAL-PDT is effective for FMF on the face and neck26. In this study, 2 patients with stage IA and lesions on the face achieved CR after undergoing MAL-PDT for a total of 2 doses (once per month). Another 2 patients in stage IA with follicular lesions on the chest and face reached CR after UVA-1 treatment; however, these patients then developed relapse. These 2 patients with relapse subsequently underwent MAL-PDT once per month (for a total of 4 times), and both achieved CR. None of the four patients aforementioned experienced any relapse over an average of 36 months of follow-up from the last treatment. MAL-PDT was performed 3 times on average, at a frequency of once per month. This treatment schedule was more frequent than that used in previous studies. The 4 patients with stage IA FMF, including the 2 patients who experienced a relapse, achieved CR. This result demonstrates MAL-PDT's excellent therapeutic effect in FMF. Methyl ester bond of MAL has great lipophilicity. This enables MAL to penetrate deeper into skin and make effect of MAL-PDT more selective for pilosebaceous unit27. In this way, MAL-PDT may be specific for FMF among MF. MAL-PDT is also advantageous considering its noninvasive nature, relative selectivity, low risk of toxicity, and excellent cosmetic outcomes. Therefore, MAL-PDT may be a good treatment option in FMF. In particular, MAL-PDT is efficacious at an early stage, in cases that are resistant to initial therapy, or in cases of relapses on a sensitive area (such as the face or neck).
References
1. Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004; 18:397–415. PMID: 15196152.

2. van Doorn R, Van Haselen CW, van Voorst Vader PC, Geerts ML, Heule F, de Rie M, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000; 136:504–510. PMID: 10768649.
3. Jang MS, Kang DY, Park JB, Kim JH, Park KA, Rim H, et al. Pityriasis lichenoides-like mycosis fungoides: clinical and histologic features and response to phototherapy. Ann Dermatol. 2016; 28:540–547. PMID: 27746631.

4. Muniesa C, Estrach T, Pujol RM, Gallardo F, Garcia-Muret P, Climent J, et al. Folliculotropic mycosis fungoides: clinicopathological features and outcome in a series of 20 cases. J Am Acad Dermatol. 2010; 62:418–426. PMID: 20079954.

5. Aydogan K, Yazici S, Balaban Adim S, Tilki Gunay I, Budak F, Saricaoglu H, et al. Efficacy of low-dose ultraviolet a-1 phototherapy for parapsoriasis/early-stage mycosis fungoides. Photochem Photobiol. 2014; 90:873–877. PMID: 24502428.

6. Lehman JS, Cook-Norris RH, Weed BR, Weenig RH, Gibson LE, Weaver AL, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010; 146:607–613. PMID: 20566923.
7. Gerami P, Rosen S, Kuzel T, Boone SL, Guitart J. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008; 144:738–746. PMID: 18559762.
8. van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002; 138:191–198. PMID: 11843638.

9. Gerami P, Guitart J. The spectrum of histopathologic and immunohistochemical findings in folliculotropic mycosis fungoides. Am J Surg Pathol. 2007; 31:1430–1438. PMID: 17721200.

10. Amitay-Laish I, Feinmesser M, Ben-Amitai D, Fenig E, Sorin D, Hodak E. Unilesional folliculotropic mycosis fungoides: a unique variant of cutaneous lymphoma. J Eur Acad Dermatol Venereol. 2016; 30:25–29.

11. Cerroni L. Skin lymphoma: the illustrated guide. 4th ed. Oxford: Wiley-Blackwell;2014.
12. Jang MS, Kang DY, Park JB, Han SH, Lee KH, Kim JH, et al. Clinicopathological manifestations of ichthyosiform mycosis fungoides. Acta Derm Venereol. 2016; 96:100–101. PMID: 26062766.

13. Hur J, Seong JY, Choi TS, Jang JG, Jang MS, Suh KS, et al. Mycosis fungoides presenting as Ofuji's papuloerythroderma. J Eur Acad Dermatol Venereol. 2002; 16:393–396. PMID: 12224701.

14. Jang MS, Kang DY, Han SH, Park JB, Kim ST, Suh KS. CD25+ folliculotropic Sézary syndrome with CD30+ large cell transformation. Australas J Dermatol. 2014; 55:e4–e8. PMID: 23190349.

15. Oppen K, Bjerner J, Buchmann M, Piehler AP. Incidental findings of monoclonal proteins from carbohydrate-deficient transferrin analysis using capillary electrophoresis. Clin Chem Lab Med. 2017; 55:e133–e136. PMID: 27816951.

16. Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010; 28:4730–4739. PMID: 20855822.
17. Benner MF, Jansen PM, Vermeer MH, Willemze R. Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood. 2012; 119:1643–1649. PMID: 22160616.

18. van Santen S, Roach RE, van Doorn R, Horváth B, Bruijn MS, Sanders CJ, et al. Clinical Staging and Prognostic Factors in Folliculotropic Mycosis Fungoides. JAMA Dermatol. 2016; 152:992–1000. PMID: 27276223.

19. Klemke CD, Dippel E, Assaf C, Hummel M, Stein H, Goerdt S, et al. Follicular mycosis fungoides. Br J Dermatol. 1999; 141:137–140. PMID: 10417530.

20. Breuckmann F, von Kobyletzki G, Avermaete A, Radenhausen M, Höxtermann S, Pieck C, et al. Mechanisms of apoptosis: UVA1-induced immediate and UVB-induced delayed apoptosis in human T cells in vitro. J Eur Acad Dermatol Venereol. 2003; 17:418–429. PMID: 12834452.
21. Weichenthal M, Schwarz T. Phototherapy: how does UV work? Photodermatol Photoimmunol Photomed. 2005; 21:260–266. PMID: 16149939.

22. Tewari A, Grage MM, Harrison GI, Sarkany R, Young AR. UVA1 is skin deep: molecular and clinical implications. Photochem Photobiol Sci. 2013; 12:95–103. PMID: 23192740.

23. Yamauchi R, Morita A, Yasuda Y, Grether-Beck S, Klotz LO, Tsuji T, et al. Different susceptibility of malignant versus nonmalignant human T cells toward ultraviolet A-1 radiation-induced apoptosis. J Invest Dermatol. 2004; 122:477–483. PMID: 15009733.

24. Olek-Hrab K, Silny W, Dańczak-Pazdrowska A, Osmola-Mańkowska A, Sadowska PA, Polańska A, et al. Ultraviolet A1 phototherapy for mycosis fungoides. Clin Exp Dermatol. 2013; 38:126–130. PMID: 23082901.

25. Kim ST, Kang DY, Kang JS, Baek JW, Jeon YS, Suh KS. Photodynamic therapy with methyl-aminolaevulinic acid for mycosis fungoides. Acta Derm Venereol. 2012; 92:264–268. PMID: 22170261.

26. Debu A, Bessis D, Girard C, Du Thanh A, Guillot B, Dereure O. Photodynamic therapy with methyl aminolaevulinate for cervical and/or facial lesions of folliculotropic mycosis fungoides: interest and limits. Br J Dermatol. 2013; 168:896–898. PMID: 23013351.

27. Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate. J Am Acad Dermatol. 2006; 54:647–651. PMID: 16546587.

Fig. 1
(A) Localized erythematous discrete plaques and multiple milia-like lesions on the face. (B) Biopsy specimens show a perifollicular cell infiltrate (H&E, ×40). (C) Small and medium to large pleomorphic lymphocytes with large cell transformation (arrows) are seen (H&E, ×200) (patient #13).
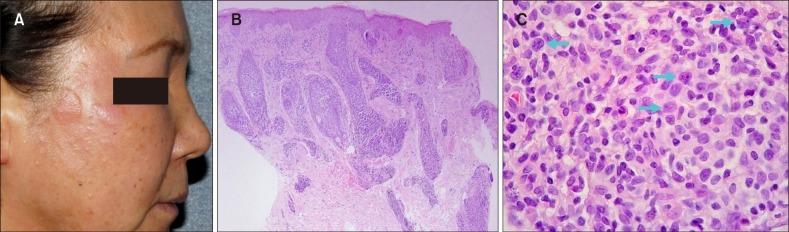
Fig. 2
(A) Papuloerythroderma with a typical sparing of the abdominal skin folds (‘deck-chair’ sign) and plaques with follicular accentuation. (B) Biopsy specimens show perifollicular infiltrate and coarse collagen bundles in the papillary dermis (H&E, ×40). (C) Folliculotropic lymphocytes and eosinophilic folliculitis are seen (H&E, ×200) (patient #8).
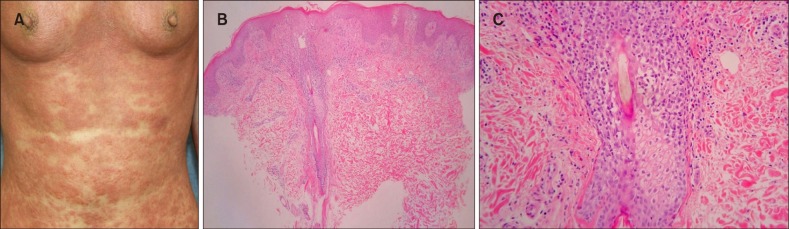
Fig. 3
(A) Agminated lesion of erythematous discrete papules on the left chest. (B) Close-up view. (C) After 4 times of methyl aminolevulinate-photodynamic therapy treatments, the skin lesions disappeared almost completely. (D) Close-up view. (E) Biopsy specimens show a large dilated follicular unit distended by keratinous material and infiltrated by lymphocytes (H&E, ×40) (patient #16).
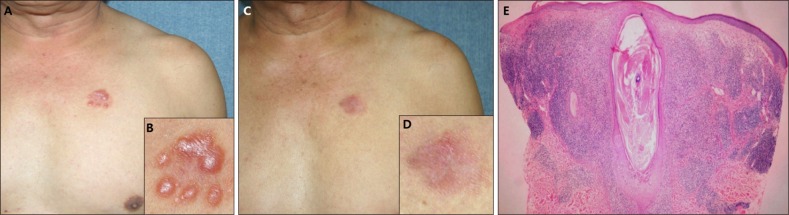
Fig. 4
(A) The facial skin with development of leonine face and scaly patches with erythroderma. (B) Biopsy specimens show a dense band-like infiltrate of lymphocytes in the upper dermis and stuffed dermal papilla with lymphocytes (H&E, ×40). (C) Follicular and perifollicular infiltrates of lymphocytes with follicular mucin are seen (H&E, ×100) (patient #9).
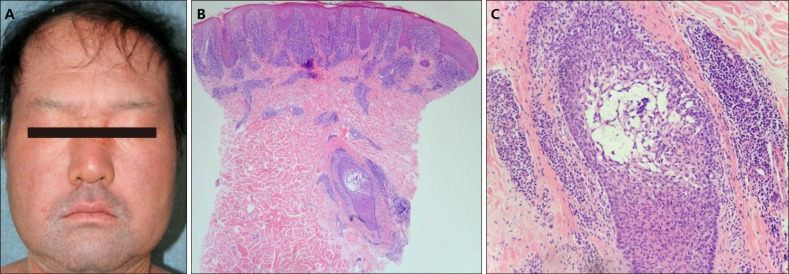
Table 1
Clinical data, treatments, outcomes, and F/U of 20 patients with FMF
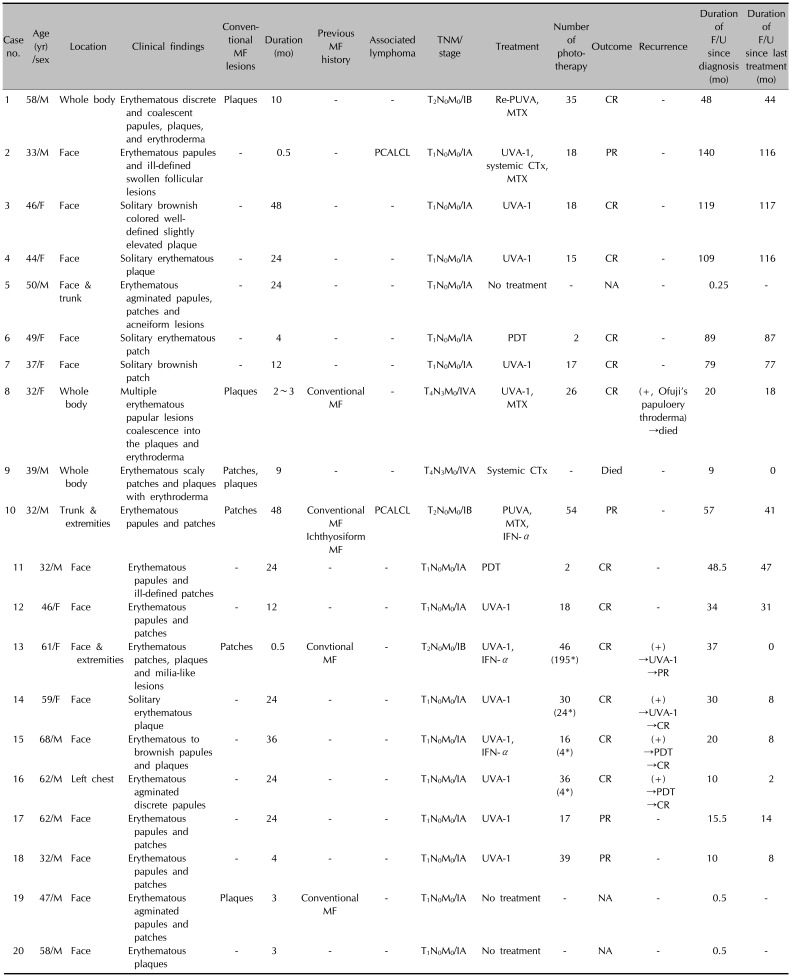
F/U: follow up, FMF: folliculotropic mycosis fungoides, MF: mycosis fungoides, -: none, M: male, F: female, re-PUVA: retinoid plus psoralen plus ultraviolet A, MTX: methotrexate, CR: complete remission, PCALCL: primary cutaneous anaplastic large-cell lymphoma, UVA-1: ultraviolet A-1 radiation, CTx: chemotherapy, PR: partial remission, PDT: photodynamic therapy, PUVA: psoralen plus UVA, IFN-α: interferon-α, NA, not available. *Number of phototherapy after recurrence.
Table 2
Summary of clinical findings of 20 patients with folliculotropic mycosis fungoides
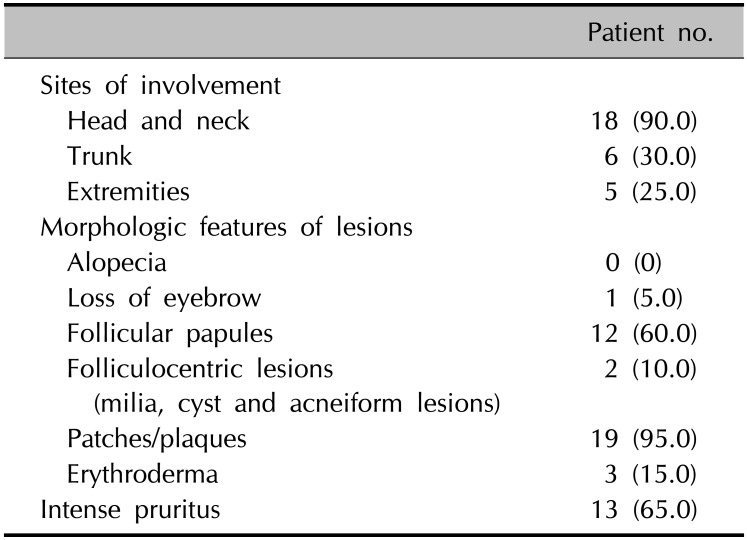
Table 3
Histopathologic findings of 20 patients with folliculotropic mycosis fungoides




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download