Abstract
Background
Reactive oxygen species (ROS) play an important role in the induction of apoptosis under pathological conditions. Recently, a significant increase in ROS production and disrupted apoptosis mechanisms in keloids have been reported. Nuclear factor erythroid 2-related factor 2 (Nrf2) represents one of the most important cellular defense mechanisms against oxidative stress and is implicated in the regulation of apoptosis. Recently, it has been reported that Nrf2 upregulates Bcl-2, an anti-apoptotic protein.
Methods
ROS generation in keloid tissues was evaluated with OxyBlot analysis. Western blotting and/or immunohistochemical staining approaches were used to study expression of Nrf2 or Bcl-2 in keloid and normal skin tissues. Cellular fractionation was performed to examine subcellular distribution of Nrf2. Transfection of fibroblasts with Nrf2-specific small interfering RNA (siRNA) was conducted to understand the relationship between Nrf2 expression and apoptosis induction.
Results
Protein oxidation, a marker of oxidative stress, is increased in keloid tissues. Western blot analysis clearly showed that Nrf2 and Bcl-2 are downregulated in keloid tissues. Immunohistochemical staining of Nrf2 confirmed the results of the western blot analysis. Transfection of fibroblasts with the Nrf2-specific siRNA results in increased apoptosis and decreased cell viability.
Conclusion
Collectively, our data indicate that Nrf2 expression is downregulated in keloid tissues, and that Nrf2 is involved in the development of apoptosis in Nrf2 siRNA-transfected fibroblasts. We propose that a defective antioxidant system and apoptotic dysregulation may participate in keloid pathogenesis.
Keloids are benign hyperproliferative growths of connective tissue and are composed of large collagen bundles that extend beyond the boundary of the original wound. They are characterized by the proliferation of dermal fibroblasts, overproduction of extracellular matrix components, and increased infiltration of inflammatory cells including lymphocytes, mast cells, and macrophages. Deposition of a dense collagenous meshwork with a rich vascular supply and increased intrusion of inflammatory cells are probably related to the proliferative and inflammatory characteristics of keloids. However, the precise pathogenic mechanisms that cause keloids remain unknown.
Previous studies have investigated the effects of various growth factors and the disruption of apoptotic mechanisms in the pathogenesis of keloids1234. A number of abnormalities in various cellular functions, including proliferation, apoptosis, or expression of growth factors and extracellular proteins, were found in fibroblasts derived from keloid tissues56. Recently, a significant increase in the generation of reactive oxygen species (ROS) was found in keloid fibroblasts, which could relate to the inflammatory and oxidative stress features of the disease7.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is one of the most important cellular defense proteins to combat oxidative stress8-10. A protective role for the Nrf2 pathway has been established in many human disorders, including cancer, inflammatory disease, and neurodegenerative disease11. Moreover, it has been reported that increased free radicals and impaired antioxidant scavenging are involved in the pathogenesis of keloids11.
As Nrf2 is a main defense mechanism against oxidative stress, a decrease in expression of Nrf2 may contribute to increased ROS production in keloid tissue. To the best of our knowledge, there have not been any studies reporting the expression of antioxidant proteins in keloids. Here, we compare Nrf2 expression in normal skin and keloid tissues using western blot and immunohistochemical analyses, and examine its roles in human fibroblast cells transfected with Nrf2-specific small interfering RNA (siRNA).
Keloid specimens were obtained from patients who had undergone surgery in the Department of Plastic and Reconstructive Surgery. The institutional review board of Soonchunhyang University Seoul Hospital reviewed and approved this research protocol involving the use of tissue samples.
Eight keloid tissues were obtained from eight female patients and were diagnosed by pathology. Normal skin tissues were collected from the backs of 8 women who had breast reconstruction with a latissimus dorsi flap (Table 1). For immunohistochemical analysis, archival formalin-fixed, paraffin-embedded tissues were used.
We used 2~3 specimens from each of the eight normal skin samples and eight keloid samples. A portion of each specimen was frozen in liquid nitrogen immediately after resection for western blotting analysis, and stored at -80℃. The remaining samples were fixed in formalin, embedded in paraffin wax, and then stored until needed for immunohistochemical studies.
The OxyBlot Protein Oxidation Detection kit (Chemicon International, Temecula, CA, USA) was used to detect carbonyl groups introduced into proteins by oxidative modification. 2,4-Dinitrophenylhydrazine (DNP) derivatization was carried out for 15 min following the manufacturer's instructions on 10 µg of protein obtained from the tissue. The DNP-derivatized protein samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene difluoride membranes, stained with Ponceau red, and then probed with an anti-DNP antibody. Blots were developed using a chemiluminescence detection system.
Tissue samples were homogenized in whole cell extract buffer [25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.7), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetra acetic acid, 0.1% Triton X-100, 0.5 mM dithiothreitol, 20 mM glycerol phosphate, 0.1 mM Na3VO4, 2 g/ml leupeptin, 2 g/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail tablet (Boehringer Mannheim, Mannheim, Germany). The tissue suspension was rotated at 4℃ for 10 min. Supernatants were collected and maintained at -70℃ for western blotting. Proteins from tissue were separated by SDS-PAGE using NuPAGE 4%~12% Bis-Tris gels (NP-0335Box; Invitrogen, Carlsbad, CA, USA) and then transferred to Immobilon-P (Sigma, St. Louis, MO, USA) membranes. The membranes were blocked with 5% bovine serum albumin in 1×Tris-buffered saline-0.1% Tween 20 (TBS-T) solution. The membranes were probed with primary antibody diluted 1:1,000 at 4℃ for 16 h. The blots were washed four times with 1×TBS-T buffer followed by treatment with secondary antibody for 1 h. After the secondary antibody reaction, the wells were washed four times. Proteins on the membrane were detected using the Enhanced Chemiluminescence Solution kit (Amersham, Buckinghamshire, UK). The membranes were stripped and reblotted with anti-actin antibody as a loading control (catalog #A5441; Sigma).
The primary antibodies used were anti-Nrf2 (SC-13032; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-Keap-1 (60027-1-Ig; Proteintech Group, Chicago, IL, USA), and anti-Bcl-2 (#2870; Cell Signaling Technology, Danvers, MA, USA). The secondary antibodies used were horseradish peroxidase-linked anti-rabbit immunoglobulin (Ig) G (catalog #7074; Cell Signaling Technology) and anti-mouse IgG (SC-2005; Santa Cruz Biotechnology Inc.).
Cytoplasmic and nuclear extracts were prepared according to the instructions of the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL, USA). Subcellular fractions of normal and keloid skin tissues were used for western blot analysis.
For immunohistochemical analysis, paraffin sections (4 µm thick) were prepared and staining was performed by the avidin-biotin complex method. In brief, slides were deparaffinized in xylene and rehydrated with ethanol. The endogenous peroxidase activity was inhibited by immersion of the slides in 3% H2O2/methanol. Antigen retrieval was performed in a microwave oven for 15 min with 10 mM citrate buffer (pH 6.0). Pre-incubation took place with a blocking solution for 30 min to prevent nonspecific binding. The sections were then incubated overnight at 4℃ with the Nrf2 antibody (SC-13032, 1:500). The slides were subsequently incubated with biotinylated secondary antibody for 30 min and then with streptavidin-peroxidase, for another 30 min. The visualization of the immunoreaction was performed with 3,3'-diaminobenzidine (DBC500; ScyTek, Logan, UT, USA). Negative controls were performed in the same way, substituting the primary antibody with phosphate buffered saline. Immunohistochemical assessment was performed by measuring the intensity of labeling (-, 1+, 2+, and 3+) and the percentage of immunohistochemically stained cells (<25%, 25% ~75%, and >75%) (Table 2). The final assessment was determined by several investigators independently.
The normal human fibroblast and G361 cell lines served as positive controls for Nrf2 expression and Bcl-2 expression, respectively. The normal fibroblast cell line was a kind gift of Dr. Jeung Hoon Lee (Chungnam National University, Daejeon, Korea). The human malignant melanoma cell line G361 was obtained from the American Type Culture Collection (CRL 1424; Virginia, MD, USA). Cells were incubated in Dulbecco's modified eagle's medium containing 10% fetal calf serum, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37℃ in a 5% CO2 incubator.
siRNA against Nrf2 and scrambled control siRNA were purchased from Invitrogen (Stealth siRNAs [Set of 3] HSS181505, HSS181506, HSS107130). The siRNA transfection was performed with Lipofectamine 2000 (catalog# 11668-019; Invitrogen), according to the manufacturer's instructions. A siRNA concentration of 80 pmol was used for a 35 mm dish of fibroblast cells.
The apoptotic cell distribution was determined using Muse Annexin V and Dead Cell kit according manufacturer's protocol (MCH100105; Merck Millipore, Billerica, MA, USA). Briefly, cells were collected by centrifugation, mixed with the Muse Annexin V and Dead Cell reagent, and analyzed using a Muse Cell Analyzer (cat. no. 0500-3115; Merck Millipore).
The cells were seeded in 96 well microtiter plates, followed by transfection with 10 nM Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h, 48 h, and 72 h, after which they were exposed to MTT (final 0.1 mg/ml) for an additional 4 h. The formazan crystals, formed by the reduction of MTT in living cells, were solubilized in 200 µl of dimethyl sulfoxide and the concentration was measured by spectrophotometry at 560 nm. The results were expressed as a percentage, based on the ratio of the absorbance between the treated cells and the controls (100%).
Data from the Raytest TINA software were analyzed using PASW Statistics ver. 17.0 (IBM Co., Armonk, NY, USA) and statistical significance was set at p<0.05. Data are presented as mean±standard deviation. We used the Mann-Whitney U test to compare non-normally distributed variables.
The OxyBlot analysis revealed increased oxidative stress in keloid tissues compared to normal skin tissues (Fig. 1A). Relative values for densitometric dinitrophenylhydrazone (DNP) derivatives in OxyBlot analysis were approximately 9.983×106 (9.401×106~1.093×107) in keloid and 7.644×106 (6.050×106~7.994×106) in normal skin tissues (p<0.05) (Fig. 1B). These results indicate that oxidative protein damage (DNP derivatives) is significantly increased in keloid tissues compared to normal skin.
Western blot analysis of subcellular fractions from keloid and normal skin tissues reveal Nrf2 with the similar quantity of expression between nuclear and cytoplasmic fractions in each sample (Fig. 2A). However, the levels of Nrf2 protein are significantly lower in keloid tissues compared to normal skin tissues (Fig. 2B), whereas Keap-1 is not expressed in either keloid or control skin tissues (data not shown). The results of the Mann-Whitney U test show a median (interquartile range) of 0.3742 (0.300~0.475) and 0.5168 (0.420~0.601) for keloid and normal skin tissues, respectively (p<0.05) (Fig. 2C).
Using immunohistochemical assessment, we confirmed that Nrf2 expression is significantly downregulated in keloid tissues compared to normal skin tissues. Fig. 3 shows representative immunohistochemical staining for Nrf2 in normal skin and keloid tissues, showing significantly stronger staining of Nrf2 in normal skin compared to keloid tissues. As shown in Table 2, much less component of cells were stained with Nrf2 in keloid than normal skin tissues.
An increase in the rate of apoptosis was found in normal human fibroblasts after transfection with Nrf2-specific siRNA (Fig. 4). Downregulation of Nrf2 expression is observed 48 h and 72 h after Nrf2-siRNA treatment by western blot analysis (Fig. 4A). During Nrf2-siRNA treatments, phase contrast images of treated and control cells were captured. After 72 h the cell shape is deeply altered and the some of the cells appeared detached (Fig. 4B). In the apoptosis assay, three different cell populations can be detected after Annexin V-PE staining of fibroblasts: live cells group in the lower left, early apoptotic cells group in the lower right, and late apoptotic cells group in the highest right part of the panel. An increase in the apoptotic cell population is found after Nrf2-siRNA treatment (Fig. 4C). Using the MTT assay, cell proliferation is decreased in a time-dependent manner after Nrf2 knockdown in fibroblasts (Fig. 4D).
Using western blot analysis, the expression of Bcl-2 is significantly lower in keloid tissues compared to normal skin tissues (Fig. 5A). The results of the Mann-Whitney U test show a median (interquartile range) of 0.266 (0.237~0.296) for keloid and 0.435 (0.359~0.490) for normal skin tissues (p<0.05) (Fig. 5B).
Keloid formation is a pathological response of wound healing to cutaneous injury in genetically susceptible individuals12. Keloids are defined as scars growing beyond the margins of the original wound that rarely regress over time13. Deposition of a dense collagenous meshwork with a rich vascular supply and increased infiltration of inflammatory cells is probably related to the proliferative and inflammatory status of keloids. There are a number of reports suggesting that oxidative stress is involved in various chronic inflammatory conditions14. In addition, oxidative damage appears to play a relevant role in the pathogenesis of various fibrotic diseases, and it has been reported that secondary production of ROS occurs after triggering inflammation due to depletion of antioxidants15. Considering these previous reports, the fibrotic and inflammatory status of keloids may be associated with ROS production. The oxidative status in keloids has been investigated in keloid fibroblasts but has not been examined in whole keloid tissues. De Felice et al.7 reported that ROS production was increased in keloid fibroblasts and suggested that this is probably involved in the pathogenesis of the disease. In our study, protein carbonyl levels, used as a marker of oxidative protein modification, were elevated in keloid tissues (Fig. 1). Our data, consistent with the findings of De Felice et al.7, suggest the presence of oxidative stress via ROS production in keloid tissues and that an impaired cellular antioxidant system may participate in this increased ROS generation.
Nrf2 comprises the main cellular antioxidant system against chemical- and radiation-induced oxidative and electrophilic stress16. Nrf2 is known to interact with actin-associated cytosolic protein, which is an inhibitor of Nrf2 (INrf2), and is also known as Kelch-like ECH-associated protein 1 (Keap1)171819202122. Keap1 functions as a substrate adaptor protein for a Cul3-Rbx1-dependent E3 ubiquitin ligase complex to ubiquitinate Nrf2, leading to its degradation and maintaining asteady state level of Nrf216. Nrf2 plays a role in cellular defense by regulating various cytoprotective proteins through Keap1-dependent or independent signaling pathways. Nrf2 is also controlled by other mechanisms that have not yet been fully elucidated. Rada et al.23 showed that degradation of Nrf2 is mediated by the Neh6 domain of mouse Nrf2 in a Keap1-independent manner. Activation of the Nrf2 defense response protects against neurodegenerative diseases, aging, diabetes, photo-oxidative stress, cardiovascular disease, inflammation, pulmonary fibrosis, acute pulmonary injury, and cancer924252627. Considering the critical role of Nrf2 against oxidative stress, a significant association between Nrf2 and increased oxidative stress in keloids may exist. However, no previous studies have investigated the role of Nrf2 expression in keloids. In our study, western blot analysis revealed that Nrf2 expression is clearly downregulated in keloid tissues compared to normal skin tissues (Fig. 2B). This result suggests that downregulation of Nrf2, the primary antioxidant system in the cell, results in the increased oxidative stress found in keloid tissues.
Ning et al.28 investigated the localization of Nrf2 in the nucleus and cytoplasm under varying concentrations of hydrogen peroxide in cultured pulmonary microvascular endothelial cells. They found that low and moderate doses of hydrogen peroxide exposure led to the nuclear accumulation of Nrf2, whereas high doses of hydrogen peroxide exposure led to nuclear exclusion of Nrf2. In our experiment, Nrf2 was found in the cytoplasmic and nuclear fractions of both normal and keloid tissues (Fig. 2A). Notably, total Nrf2 expression was lower in keloid tissues compared with that in normal tissues, while the quantity of Nrf2 expression was similar between the nucleus and cytoplasm in each sample. However, Keap1 was not detected in either normal or keloid skin tissues in the western blot (data not shown), suggesting that Nrf2 might be regulated by a Keap1-independent pathway in skin tissues. In our immunohistological analysis, much less component of cells were stained with Nrf2 in keloid than normal skin tissues (Fig. 3), which was consistent with the result of western blot analysis. These results indicate that dysregulation of ROS and Nrf2 levels may be involved in the pathogenesis of keloids.
There is growing evidence suggesting that oxidative stress resulting from ROS production is implicated in apoptosis and that excessive production of ROS results in both DNA and protein damage2930. As ROS can contribute to the induction of apoptosis, especially in inflammatory cells, the inflammatory and increased oxidative status of keloids may affect the apoptotic activity in keloid31. A few studies have attempted to explain the etiology of keloids and have reported abnormalities in the regulation of apoptosis during the wound healing process. They have suggested that pathological scarring might be a consequence of the accumulation of granulation tissue as a result of defective apoptosis mechanisms32333435. Desmoulière et al.33 have shown that the number of apoptotic cells sharply increases as the wound closes, suggesting that apoptosis is involved in the progressive transformation of granulation tissue into scar.
A few studies have reported increased apoptosis in keloid fibroblasts536. Akasaka et al.37 reported increased apoptosis in keloid fibroblast cells compared to normal fibroblasts and hypertrophic scars. Several proteins involved in apoptosis in keloid fibroblasts have also been identified. Jiménez et al.38 indicated that c-myc and rho-p21 are potent inducers of apoptosis, whereas Bcl-2, v-abl, E1B, and ras prevent activation of the apoptotic pathway in fibroblasts. Akasaka et al.36 also reported that the increased expression of caspase-3 in keloids can induce apoptosis in fibroblasts. Since ROS production can induce apoptosis, we hypothesized that Nrf2 may be involved in this process. Recent studies have suggested the possibility of an association between Nrf2 and apoptosis. In a study by Pan et al.39, an increase in apoptotic rate was found in human glioblastoma U251 cells transfected with Nrf2-specific siRNA. Consistent with these previous studies, we found that Nrf2 knockdown by siRNA produced growth-inhibiting and apoptosis- activating effects infibroblasts (Fig. 4). These data indicate that the downregulation of Nrf2 may play a role in activation of apoptotic pathways in keloid tissues.
Niture and Jaiswal40 found that Nrf2 upregulates expression of the anti-apoptotic protein Bcl-2, which leads to a reduction in cellular apoptosis in vitro. From this, we postulated that decreased expression of Nrf2 in keloid tissues may result in downregulation of Bcl-2. Indeed, we observed decreased expression of Bcl-2 in keloid tissues compared to normal skin tissue (Fig. 5). Although we did not explore the possibility of crosstalk between Nrf2 and Bcl-2, the downregulation of Bcl-2 may participate in increased apoptotic activity in keloid tissues.
These results support the previous notion that apoptosis increases in keloids and that this apoptotic dysregulation may play a crucial role in development of pathologic scarring. Given that apoptosis rates increased when Nrf2 was knocked down, we suggest that Nrf2 could be one of the central proteins involved in the pathogenesis of keloids.
To our knowledge, this is the first study to investigate the role of Nrf2 protein expression in keloid tissues. In summary, our data suggest that downregulation of Nrf2 expression in keloids could cause increased oxidative damage and lead to apoptosis in fibroblasts, which may participate in keloid pathogenesis.
Figures and Tables
Fig. 1
Measurements of relative values for dinitrophenylhydrazine (DNP) derivatives in the oxyblot analysis of normal skin and keloid tissues. (A) The OxyBlot Protein Oxidation Detection kit (S7150; Millipore, Billerica, MA, USA) was used to detect overall carbonyl groups introduced into the protein side chains by oxidative modification. (B) The median values of normal skin and keloid tissues were measured by the Mann-Whitney test (n=8, *p<0.05).
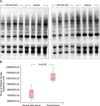
Fig. 2
Nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression in the nucleus and cytoplasm of normal skin and keloid tissues. (A) Nrf2 was seen in the nuclear (N) and the cytoplasmic (C) fraction of normal skin tissues and keloid tissues. β-actin (a control for cytoplasmic fractionation) and Lamin A/C (a control for nuclear fractionation) were detected by western blotting in the fractions. (B) Western blot analysis. Nrf2 expression was low in keloid tissues. β-actin used as a loading control. The human fibroblasts served as a positive control for Nrf2 expression. (C) The median values of normal skin and keloid tissues were measured by the Mann-Whitney test (n=8, *p<0.05).
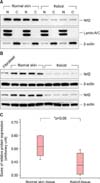
Fig. 3
Representative immunohistochemistry staining for nuclear factor erythroid 2-related factor 2 protein expression in (A, B) paraffin-embedded normal skin tissue and (C, D) keloid tissue. (A) Strongly positive staining in keratinocyte of epidermis (×100). (B) Strongly positive staining in nucleus and cytoplasm of keratinocyte of epidermis (×400). (C) Weakly positive staining in keratinocyte of epidermis (×100). (D) Weakly positive staining in nucleus and cytoplasm of keratinocyte of epidermis (×400).
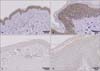
Fig. 4
Confirmation of cell viability in human fibroblast after transfection with nuclear factor erythroid 2-related factor 2 (Nrf2)-specific small interfering (siRNA). (A) Cells were transfected with 10 nM of Nrf2-targeting siRNA (siNrf2) or Stealth RNAi control (siC) for 24 h and 48 h, after which the levels of Nrf2 were measured by Western blot analysis. (B) Phase contrast images of cells treated with Nrf2-siRNA and control cells. (C) Annexin V-binding assay. 7-Aminoactinomycin D (7-AAD), phycoerythrin (Annexin V-PE). (D) Effects of Nrf2-siRNA silencing on proliferation of the fibroblasts.
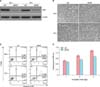
Fig. 5
Bcl-2 expression in normal skin and keloid tissues. (A) Western blot analysis. Bcl-2 expression was low in keloid tissues. β-actin used as a loading control. The G361 cell line served as a positive control for Bcl-2 expression. (B) The median values of normal skin and keloid tissues were measured by the Mann-Whitney test (n=8, *p<0.05).

References
1. Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989; 9:1642–1650.

2. Haisa M, Okochi H, Grotendorst GR. Elevated levels of PDGF alpha receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol. 1994; 103:560–563.

3. Oliver N, Babu M, Diegelmann R. Fibronectin gene transcription is enhanced in abnormal wound healing. J Invest Dermatol. 1992; 99:579–586.

5. Appleton I, Brown NJ, Willoughby DA. Apoptosis, necrosis, and proliferation: possible implications in the etiology of keloids. Am J Pathol. 1996; 149:1441–1447.
6. Funayama E, Chodon T, Oyama A, Sugihara T. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Invest Dermatol. 2003; 121:1326–1331.

7. De Felice B, Garbi C, Santoriello M, Santillo A, Wilson RR. Differential apoptosis markers in human keloids and hypertrophic scars fibroblasts. Mol Cell Biochem. 2009; 327:191–201.

8. Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999; 31:319–324.

9. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007; 47:89–116.

10. Lee YJ, Jeong HY, Kim YB, Lee YJ, Won SY, Shim JH, et al. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem Toxicol. 2012; 50:116–123.

11. Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012; 32:687–726.

12. Park SY, Park JY, Kim CH, Kang SU, Kim JH, Bark KM, et al. Effects of xanthium stramarium and psoralea corylifolia extracts combined with UVA1 irradiation on the cell proliferation and TGF-β1 expression of keloid fibroblasts. Ann Dermatol. 2013; 25:304–309.

13. Son IP, Park KY, Kim B, Kim MN. Pilot study of the efficacy of 578 nm copper bromide laser combined with intralesional corticosteroid injection for treatment of keloids and hypertrophic scars. Ann Dermatol. 2014; 26:156–161.

14. Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004; 10:1647–1652.

15. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992; 119:598–620.
16. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009; 47:1304–1309.

17. Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011; 85:241–272.

18. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997; 236:313–322.

19. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the aminoterminal Neh2 domain. Genes Dev. 1999; 13:76–86.

20. Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc). 2006; 71:962–974.

21. Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004; 37:433–441.

22. Stewart JD, Hengstler JG, Bolt HM. Control of oxidative stress by the Keap1-Nrf2 pathway. Arch Toxicol. 2011; 85:239.

23. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011; 31:1121–1133.

24. Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006; 8:99–106.

25. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008; 58:262–270.

26. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004; 10:549–557.

27. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006; 38:769–789.

28. Ning JL, Mo LW, Lai XN. Low- and high-dose hydrogen peroxide regulation of transcription factor NF-E2-related factor 2. Chin Med J (Engl). 2010; 123:1063–1069.
29. Greenlund LJ, Deckwerth TL, Johnson EM Jr. Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995; 14:303–315.

30. Higuchi M, Honda T, Proske RJ, Yeh ET. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene. 1998; 17:2753–2760.

31. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–418.
32. Desmoulière A, Badid C, Bochaton-Piallat ML, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997; 29:19–30.

33. Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995; 146:56–66.
34. Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998; 30:1019–1030.

35. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989; 84:827–837.
36. Akasaka Y, Ishikawa Y, Ono I, Fujita K, Masuda T, Asuwa N, et al. Enhanced expression of caspase-3 in hypertrophic scars and keloid: induction of caspase-3 and apoptosis in keloid fibroblasts in vitro. Lab Invest. 2000; 80:345–357.

37. Akasaka Y, Ito K, Fujita K, Komiyama K, Ono I, Ishikawa Y, et al. Activated caspase expression and apoptosis increase in keloids: cytochrome c release and caspase-9 activation during the apoptosis of keloid fibroblast lines. Wound Repair Regen. 2005; 13:373–382.

38. Jiménez B, Arends M, Esteve P, Perona R, Sánchez R, Ramón y, et al. Induction of apoptosis in NIH3T3 cells after serum deprivation by overexpression of rho-p21, a GTPase protein of the ras superfamily. Oncogene. 1995; 10:811–816.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download