INTRODUCTION
Myopericytoma (MP) is one of a family of benign tumors showing a myoid/pericytic line of differentiation. It is composed of oval to spindle-shaped myoid-appearing cells with a striking tendency for concentric perivascular growth1. It is characterized by a well-circumscribed, slow-growing painless mass that arises most commonly in the dermis or subcutaneous tissue of extremities in adults2,3. In the Korean dermatologic literature, previously reported articles assumed to be related to the disease were cases that occurred in the forearm, infraorbital area and auricle of the ear4-6. Unlike previous reports, this is a typical case arising in a lower extremity, which is the common clinical feature of MP.
CASE REPORT
A 45-year-old woman presented with a 2-year history of a slow-growing painless nodule in the subcutaneous tissue of the posterior side of her right lower leg. She did not have underlying disease and trauma history. Upon physical examination, a 0.9×0.7 cm sized, skin-colored firm mass on the posterior side of the right lower leg was observed (Fig. 1). We presumed this lesion as an epidermal cyst, pilomatricoma or calcinosis cutis and performed excisional biopsy.
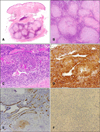
Microscopically, the tumor was composed of a myxoid matrix and spindle-shaped myoid-appearing cells with a concentric arrangement of cells around numerous blood vessel walls (Fig. 2A~C). In the intervascular area, the lesional spindle cells were present as sheets of cells. Mitotic figures were inconspicuous in the tumor. Nuclear atypia and necrosis were not observed. The lesional spindle cells were diffusely positive for smooth muscle actin (SMA) (Fig. 2D). In contrast, the tumor cells were negative for CD34, desmin and S100 protein (Fig. 2E, F). From these findings, we diagnosed this lesion as a myopericytoma.
DISCUSSION
MP is a recently described benign tumor composed of oval to spindle-shaped myoid-appearing cells that undergo concentric perivascular growth2,3. The histologic spectrum of MP is very wide and varies from lesions that are very similar to myofibromatosis to tumors that closely resemble glomus tumors and angiomyoma7. MP is composed of a mixture of solid cellular areas intermixed with variable numbers of vascular channels1,2,7-9. The cells in the solid areas are oval to spindle-shaped with eosinophilic cytoplasm and vesicular nuclei1,2,9. The vascular channels are often elongated and display prominent branching resulting in a hemangiopericytoma like appearance1,2,7,9. A common and striking feature is the presence of concentric layers of tumor cells around vascular channels resulting in a typical onion ring appearance2,7. Small foci were reminiscent of myofibroma, being composed of spindle cells with abundant eosinophilic cytoplasm embedded in a myxoid matrix organized in fasicles or whorls, and these areas bulged and could be invaginated into the lumina of intralesional blood vessels2. The lesional spindle cells in MP were diffusely positive for SMA and negative for CD34. In addition, the tumor cells remained unlabelled for desmin, suggesting a less-differentiated smooth muscle phenotype. They were also negative for cytokeratin and S100 protein, usually positive in nodular hidradenomas2,9. The novel concept of the existence of myopericytes was originally proposed by Dictor et al.10 in a report describing a tumor that involved the thyroid gland of a 5-year-old boy. This tumor showed histologic features reminiscent of myofibromatosis and hemangiopericytoma. Based on immunohistochemical analysis and electron microscopy, Dictor et al.10 proposed that the lesional cells included a population of cells that they termed 'myopericytes'. The authors also suggested that myopericytes were the constituent cells in infantile myofibromatosis. The term myopericytoma was adopted by Granter et al.3 in 1998 to describe a tumor that was closely related to myofibroma, with a distinctive perivascular arrangement of lesional oval to spindle cells in a concentric multi-layered pattern. MP has also been proposed as the term to encompass the entities myofibromatosis, adult myofibroma, glomangiopericytoma and infantile haemangiopericyoma2,3. These tumors often show overlapping histologic features and are believed to be part of a spectrum of lesions that show apparent differentiation towards myopericytes2.
The most common anatomic setting for this tumor is the skin and superficial soft tissues of adult patients. The distal extremities are frequently involved, but with increased recognition, a wider distribution has been described1,9. In a comprehensive study (54 cases), Mentzel et al.9 found that the lower extremities were most commonly affected, followed by the upper extremities, the head and neck region, and the trunk. The most common presentation is a well-circumscribed and slow-growing painless nodule, although occasional cases are painful2,9. In our case, the patient had a painless, slowly growing nodule in the subcutaneous tissue of the lower leg.
Most cases of MP are benign lesions, although a few recurring and/or malignant cases have been described7,11-13. It seems that the clinical outcome of rare malignant myopericytoma is strongly associated with the depth of the neoplasm. However, more cases with expanded follow-up have to be studied to substantiate this hypothesis12.
The differential diagnosis include glomus tumors, angioleiomyomas and nodular hidradenomas2,7,9,13. Perivascular arrangement of cells can be seen in glomus tumors. However, a concentric arrangement of cells that accentuates blood vessel walls is characteristic of MP, it is not seen in glomus tumors2. In addition, areas with spindle cells and abundant eosinophilic cytoplasm that mimic myofibroma are not seen in glomus tumors2.
Angioleiomyomas are tumors composed of mature smooth muscle cell bundles with abundant vascular channels13. These tumors are often painful and generally have a more fascicular pattern than myopericytomas2,14. They are composed of fascicles of smooth muscle cells with cigar-shaped nuclei and abundant brightly eosinophilic cytoplasmthat stain diffusely SMA and they frequently (80~90% of cases) show desmin immunoreactivity in the smooth muscle bundles2,13,14. In contrast, MP has been only rarely reported to be focally immunoreactive for desmin (9% of cases), suggesting MP is composed of immature cells than angioleiomyomas2,13. In some nodular hidradenomas, lesional epithelial cells can show a striking concentric arrangement of cells around small ducts. However, close inspection confirms that the spaces are small ducts and not blood vessels and, if any doubt persists, a cytokeratin stain should confirm the epithelial nature of the cells2,15.
MP is a rare benign tumor characterized by spindle-shaped myoid-appearing cell with a concentric arrangement in distended vessels. The differential diagnosis of lesions includes a number of tumors that can have a perivascular arrangement such as glomus tumors and angioleiomyomas. MP is a recently delineated benign neoplasm, so we believe this case will aid other physicians in recognizing this unique entity and improve the understanding of MP.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download