Abstract
Cordycepin exerts neuroprotective effects against excitotoxic neuronal death. However, its direct electrophysiological evidence in Alzheimer's disease (AD) remains unclear. This study aimed to explore the electrophysiological mechanisms underlying the protective effect of cordycepin against the excitotoxic neuronal insult in AD using whole-cell patch clamp techniques. β-Amyloid (Aβ) and ibotenic acid (IBO)-induced injury model in cultured hippocampal neurons was used for the purpose. The results revealed that cordycepin significantly delayed Aβ + IBO-induced excessive neuronal membrane depolarization. It increased the onset time/latency, extended the duration, and reduced the slope in both slow and rapid depolarization. Additionally, cordycepin reversed the neuronal hyperactivity in Aβ + IBO-induced evoked action potential (AP) firing, including increase in repetitive firing frequency, shortening of evoked AP latency, decrease in the amplitude of fast afterhyperpolarization, and increase in membrane depolarization. Further, the suppressive effect of cordycepin against Aβ + IBO-induced excessive neuronal membrane depolarization and neuronal hyperactivity was blocked by DPCPX (8-cyclopentyl-1,3-dipropylxanthine, an adenosine A1 receptor-specific blocker). Collectively, these results revealed the suppressive effect of cordycepin against the Aβ + IBO-induced excitotoxic neuronal insult by attenuating excessive neuronal activity and membrane depolarization, and the mechanism through the activation of A1R is strongly recommended, thus highlighting the therapeutic potential of cordycepin in AD.
Alzheimer's disease (AD) is an age-related neurodegenerative disease of the central nervous system (CNS) with progressive cognitive impairment. It is the most common type of senile dementia, accounting for 65%–70% of cases [12]. By the middle of the century, the number of people suffering from this disease in the United States is expected to be 13.8 million, resulting in a serious burden on public health and society [2]. AD is characterized by a loss of neuropil and development of extracellular plaques and intracellular neurofibrillary tangles [13]. In the last few decades, excitotoxicity has been found to contribute to neuronal degeneration in many acute and chronic CNS diseases, such as ischemic stroke, epilepsy, and AD [45]. A previous study also reported that excessive neuronal membrane depolarization and neuronal hyperactivity were the main causes of excitotoxic neuronal death [6]. Acute excitotoxicity is thought to be mediated by excessive neuronal membrane depolarization. This results in an osmotic imbalance when countered by an influx of Na+, Cl−, and water, eventually leading to the rupture of cell membranes [7]. Many studies demonstrated that neuronal hyperactivity was also directly associated with β-amyloid (Aβ), and that restored balanced activity ameliorated Aβ toxicity [89]. Ibotenic acid (IBO), a glutamate analog excitotoxin, activated the NMDA receptor [101112]. Previous studies confirmed that Aβ increased neuronal sensitivity to excitotoxic insults [13]. Thus, to better mimic the excitotoxic neuronal insults of AD, a low dose of excitotoxin, IBO, was administered along with Aβ into the primary cultured hippocampal neurons in the present study.
Cordycepin is a natural herbal functional component isolated from Cordyceps militaris. It possesses a wide range of biological effects, including anti-tumor, anti-diabetic, anti-oxidant, and anti-inflammatory activities [141516]. Recent evidence suggests that cordycepin can exert neuroprotective effects on the CNS [171819]. Treatment with cordycepin can help recover from neuronal death caused by lipopolysaccharide-induced microglial activation via the anti-inflammatory effects [18]. Cheng et al. [19] found that cordycepin could decrease the excitotoxicity of excitatory amino acids and block free radicals in cerebral ischemia/reperfusion injury. Additionally, cordycepin has been reported to exert suppressive effects against hypoxia-induced membrane depolarization on hippocampal CA1 pyramidal neurons, improving the neuronal electrophysiological function [20]. These results indicate that cordycepin has a protective effect on the CNS against excitotoxic neuronal damage via inhibiting the neuronal hyperexcitability. A previous study also found that cordycepin reduced glutamate-induced neuronal excitotoxicity via an adenosine A1 receptor (A1R) [5]. As one of the adenosine receptor subtypes, A1R is mainly expressed in the CNS, especially in the cortex, cerebellum, thalamus, and hippocampus [21]. Thus, it has been speculated that A1Rs are involved in regulating the CNS function. Cordycepin (3′-deoxyadenosine) is an adenosine analog. The structure of cordycepin comprises a purine (adenine) nucleoside molecule attached to a ribose sugar (ribofuranose) moiety via a β-N9-glycosidic bond. These characteristics indicate that the protective effect of cordycepin on the CNS may be closely associated with A1R. A recent study has confirmed the speculation that cordycepin exerts neuroprotective effects by inhibiting Aβ-induced hippocampal neuronal apoptosis via activating A1R [22].
Excessive neuronal membrane depolarization and neuronal hyperactivity are the main causes of excitotoxic neuronal death [6]. Therefore, in the present study, a combination of Aβ and IBO was first applied to primary cultured hippocampal neurons to mimic the excitotoxic neuronal damage model of AD. Then, the neuroprotective effect of cordycepin and its underlying mechanisms were detected by evaluating the neuronal membrane depolarization and neuronal action potential (AP) firing using whole-cell patch clamp techniques.
Aβ25–35 was dissolved in DMSO to a stock concentration of 4 mM, and a concentration of 10 µM Aβ25–35 was employed in the present study. The short fragment represented the core functional domain of the full-length Aβ peptide that self-assembled to form a predominantly β-sheet structure [23]. Therefore, Aβ25–35, instead of full-length Aβ, was used in this study to investigate the neurotoxic properties of Aβ in vitro. IBO was dissolved in DMSO to a stock concentration of 60 mM, and a final concentration of 0.15 mM IBO was used. Cordycepin was dissolved in an external solution to a stock concentration of 1.2 mM, and a final concentration of 0.2 mM cordycepin was used. DPCPX (A1R-specific blocker) was dissolved in DMSO to a stock concentration of 2 mM, and a final concentration of 1 µM DPCPX was used. Caffeine (adenosine A2A receptor (A2AR) antagonist) was dissolved in DMSO to a stock concentration of 2 mM, a final concentration of 1 µM caffeine was used. The components of the external solution for electrophysiological recordings were as follows: 117 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, and 10 mM D-glucose. The pH level was adjusted to 7.4 using NaOH. The patch pipette solution for recording comprised the following: 140 mM K-gluconate, 2 mM MgCl2, 2 mM CaCl2, 2 mM Na-ATP, 0.2 mM Na-GTP, 5 mM EGTA, and 10 mM HEPES. The internal solutions were adjusted to a pH of 7.3 using KOH. Cordycepin with 98% purity was provided by South China Normal University, and other drugs were purchased from Sigma–Aldrich (MO, USA).
The care and use of animals and the experimental protocol of this study were approved by the Institutional Care and Use Committee of Jiangxi Science and Technology Normal University (approval no. SCXK2013-0004). Primary hippocampal neurons were prepared from postnatal day 0–2 Sprague–Dawley rats, as described in a previous study with some modifications [24]. Briefly, the hippocampus was transferred to the dissociation buffer containing calcium- and magnesium-free Hank's balanced salt solution and subsequently digested with 0.025% trypsin–EDTA for approximately 15 min at 37℃, followed by trituration with pipettes in the plating media (DMEM with 10% fetal bovine serum and 25 µg/ml penicillin/streptomycin). The cells were rinsed twice, counted, and plated onto glass coverslips precoated with 0.1 mg/ml poly-D-lysine. They were cultured for 6 h, following which the medium was changed to the neuronal culture medium (neurobasal medium containing 2 mM glutamine supplement, 2% B27, and 12 µg/ml penicillin/streptomycin). Further, half of the medium was changed twice a week. All the cells were grown at 37℃ in 5% CO2, and grown again for 10 more days before starting the experiments. Unless otherwise mentioned, all the media and supplements used for cell culture were obtained from Gibco (Carlsbad, CA, USA).
The membrane potential and the neuronal AP firing were recorded using the conventional whole-cell patch clamp technique under current-clamp configurations. The recording electrodes were fabricated from borosilicate glass pipettes (Sutter Instruments, Novato, CA, USA) using a Flaming–Brown puller (P-97; Sutter Instruments). The resistance of the recording electrodes was 3–5 MΩ. The recorded neuronal cells were allowed to stabilize for approximately 3 min after the rupture of patches. Data were acquired using MultiClamp 700B (Axon Instruments, Foster City, CA, USA) and digitized using Digidata 1440A (Axon Instruments) at a sampling frequency of 10 kHz. All experiments were conducted at room temperature (22℃–25℃).
The CA1 pyramidal neurons with a resting membrane potential more negative than −60 mV were used for the study. pClamp 10.4 (Axon Instruments) and Origin Pro 8.0 (Origin Lab, Northampton, MA, USA) were used for data acquisition and analysis.
The electrophysiological parameters were measured as described in previous studies [202526]. The latency of rapid depolarization was measured from the time of drug application to the time of the onset of rapid depolarization, which was estimated by speculating the slope of rapid depolarization to the slope of slow depolarization. The onset time given in Table 1 was the time of drug application to the time of the onset of slow depolarization. The threshold point given in Table 2 was the point at which the initial slow depolarization transitioned to a more rapid depolarization. The endpoint of rapid depolarization presented the peak potential of rapid depolarization (Table 2). The amplitude (Table 2) was measured between the threshold potential and the peak potential of rapid depolarization. The time between the onset of the stimulus and the first evoked AP was measured as the evoked AP latency. The AP duration was measured as the AP width at the threshold. The amplitude of the fast afterhyperpolarization (fAHP) was measured as the deviation from the beginning of the threshold potential ≤ 5 ms after the peak of a single AP.
The results were presented as mean ± standard error of mean. The statistical significance of the difference was calculated using the two-tailed Student t-test (comparison between two groups) or the least significant difference test (multiple-comparison test). A level of confidence of p < 0.05 was used for statistical significance.
The effects of drugs on the changes in the membrane potential of cultured hippocampal neurons were recorded at zero holding current before and after drug application. The rats were divided into the following groups: control (without drug treatment); Aβ + IBO; Cor (coapplication of Aβ + IBO and cordycepin); DPCPX (DPCPX, cordycepin, and Aβ + IBO); and caffeine (caffeine, cordycepin, and Aβ + IBO). In the control group, the resting membrane potential was steady during recording. Slow depolarization and rapid depolarization were observed in the other four groups (Fig. 1). In the Aβ + IBO group, the onset time of slow depolarization was 14.5 ± 1.4 sec. The amplitude of slow depolarization was 4.52 ± 0.41 mV, and the slow depolarization lasted for 12.9 ± 2.1 sec with a positive slope of 0.42 ± 0.06 mV/s. Cordycepin extended the onset time (57.0 ± 7.0 sec) and duration (31.1 ± 4.2 sec) and reduced the slope of slow depolarization (0.16 ± 0.06 mV/s) significantly compared with the Aβ + IBO group (p < 0.01). The protective effects of cordycepin to delay the Aβ + IBO-induced neuronal membrane slow depolarization were blocked by pretreatment with DPCPX in terms of onset time (15.7 ± 1.4 sec), duration (13.8 ± 1.4 sec), and slope of slow depolarization (0.37 ± 0.05 mV/s) compared with the Cor group (p < 0.01). In the caffeine group, no significant difference in the onset time (62.4 ± 10.9 sec), duration (35.0 ± 5.9 sec), and slope of slow depolarization (0.12 ± 0.03 mV/s) was found compared with the Cor group (Table 1).
In the Aβ + IBO group, slow depolarization reached a threshold and then a rapid depolarization was initiated. The latency of rapid depolarization was 27.5 ± 3.8 sec, the threshold at which rapid depolarization was triggered was −58.1 ± 1.3 mV, the amplitude was 35.2 ± 3.17 mV, and the slope was 2.25 ± 0.22 mV/s.
In the Cor group, the latency (93.2 ± 9.7 sec) and the slope of rapid depolarization (1.08 ± 0.12 mV/s) decreased significantly compared with those in the Aβ + IBO group (p < 0.01). Interestingly, pretreatment with DPCPX (A1R-specific blocker), not with caffeine (A2AR antagonist), completely blocked the delayed action of cordycepin on Aβ + IBO-induced rapid neuronal membrane depolarization (Fig. 1 and Table 2).
The suppressive effects of cordycepin on the Aβ + IBO-induced excitotoxic neuronal insult was further determined by examining the evoked AP firing using a depolarizing stimulus of 20 pA amplitude for a duration of 600 ms (Fig. 2A). As shown in Fig. 1 and Tables 1 and 2, the pretreatment of drugs (Aβ + IBO) was set for 20 sec to avoid the occurrence of rapid depolarization (neurons with rapid depolarization were discarded). Pretreatment with DPCPX was given before the recording. The results showed that the frequency of repetitive firing significantly increased to 163.9% ± 7.8% of the control after the 20 sec addition of Aβ + IBO, compared with the control group (p < 0.01) (Fig. 2C). This was coupled with membrane depolarization from −63.2 ± 1.04 mV to −58.5 ± 1.09 mV (Fig. 2B). Moreover, in the Aβ + IBO group, the evoked AP latency markedly decreased to 68% ± 6.1% of the control (Fig. 2D), indicating that the hyperactivity of the evoked AP coupled with membrane depolarization was also induced by Aβ + IBO. This was because the decreased evoked AP latency accelerated the firing of APs.
In the Cor group, the amplitude of membrane depolarization and the frequency of repetitive AP firing decreased significantly compared with the Aβ + IBO group (p < 0.01) (Fig. 2B, C). Moreover, the evoked AP latency markedly increased compared with the Aβ + IBO group (Fig. 2D), indicating that the hyperactivity of the Aβ + IBO-induced evoked AP could be suppressed with the coapplication of cordycepin. Pretreatment with DPCPX (A1R-specific blocker), not with caffeine (A2AR antagonist), completely blocked the suppressive effect of cordycepin on the hyperactivity of the Aβ + IBO-induced evoked AP. The frequency of repetitive AP firing significantly increased to 156.1% ± 8.7% and evoked AP latency decreased to 71.0% ± 6.0% of the control, compared with the Cor group (p < 0.01) (Fig. 2).
The study further tested the suppressive effect of cordycepin on the Aβ + IBO-induced hyperactivity using a ramp depolarizing stimulus pattern, where the intensity was increased from 0 to 500 pA in a linear manner within 1,500 ms (Fig. 3A). The results showed that Aβ + IBO significantly decreased the evoked ramped AP latency to 66.2% ± 4.2% of the control (p < 0.01), and the membrane potential was depolarized from −61.6 ± 1.0 mV to −57.7 ± 1.2 mV (Fig. 3B, C). A decrease in the evoked ramped AP latency indicated that a lower-strength depolarizing stimulation was required to evoke AP firing. Consistent with the effects displayed in Fig. 2, the Aβ + IBO-induced evoked hyperactivity on AP firing was markedly suppressed by cordycepin, which was confirmed by the result obtained in the Cor group (Cor group: the evoked ramped AP latency was 95.7% ± 5.4% of the control coupled with the stable membrane potential) (p < 0.01, compared with the Aβ + IBO group) (Fig. 3). Further, the suppressive effect of cordycepin on the Aβ + IBO-induced evoked hyperactivity was completely blocked by DPCPX (A1R-specific blocker) (Fig. 3). These results suggested that cordycepin suppressed the hyperactivity of the Aβ + IBO-induced evoked AP firing, confirming again that cordycepin provided neuronal protection against the excitotoxicity insult, and the mechanism through the activation of A1R is strongly recommended.
The neuronal intrinsic properties, such as the AP spike width and the amplitude of fAHP, are closely related to the neuronal intrinsic excitability and important for regulating AP spike frequency [2026]. Thus, changes in the AP spike width and the amplitude of fAHP were analyzed in different groups after drug application. The single AP was elicited with a 10-ms brief depolarizing current pulse of 200 pA on cultured neurons before and after 20-sec drug application (Fig. 4A). The amplitude of fAHP significantly reduced after the Aβ + IBO application (67.3% ± 4.8% of the control) and the membrane potential was depolarized from −60.7 ± 1.8 mV to −56.9 ± 1.7 mV compared with the control group (p < 0.01). However, the Aβ + IBO application did not alter the AP spike width (96.3% ± 5.92% of the control) (Fig. 4). The results also showed that the Aβ + IBO application reduced the amplitude of fAHP, which was blocked by cordycepin (96.2% ± 6.6% of the control), coupled with the depolarization of a stable membrane potential from −62.9 ± 0.9 mV to −62.1 ± 1.1 mV, compared with the Aβ + IBO group (p < 0.01). The suppressive effect of cordycepin on Aβ + IBO-induced reduction in the amplitude of fAHP and the depolarization of membrane potential could be completely blocked using DPCPX (A1R-specific blocker) (Fig. 4).
This study found that cordycepin significantly delayed the Aβ + IBO-induced excessive neuronal membrane depolarization. Cordycepin increased the onset time/latency, extended the duration, and reduced the slope in both slow and rapid depolarization (Fig. 1 and Tables 1 and 2). Additionally, cordycepin reversed the neuronal hyperactivity in the Aβ + IBO-induced evoked AP firing, including the increase in repetitive firing frequency, shortening of evoked AP latency, decrease in the amplitude of fAHP, and increase in membrane depolarization (Figs. 2, 3, 4). Further, the study also found that the suppressive effect of cordycepin against Aβ + IBO-induced excessive neuronal membrane depolarization and neuronal hyperactivity could be blocked using DPCPX (A1R-specific blocker) (Tables 1 and 2 and Figs. 1, 2, 3, 4). Collectively, these results revealed the suppressive effects of cordycepin against Aβ + IBO-induced excessive neuronal membrane depolarization and neuronal hyperactivity, and the mechanism through the activation of A1R is strongly involved.
The excessive neuronal membrane depolarization and neuronal hyperactivity are the main causes of excitotoxic neuronal death [6]. Excitotoxicity contributes to neuronal degeneration in many acute and chronic CNS diseases, such as AD [45]. Thus, preventing this membrane depolarization and neuronal hyperactivity can help avoid excitotoxic neuronal death, thereby attenuating AD impairment. This hypothesis was confirmed using AD animal models. Elevated excitability and hyperactivity in the hippocampus are key contributors to reduced memory function in aging and cognitive impairment, and their natural absence in aged cohorts is prodromal to AD [27282930]. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment, including beneficial effects on synaptic dysfunction and reduction in β-amyloid (Aβ) deposition [3031]. Interestingly, the present study found that the application of cordycepin significantly delayed neuronal membrane depolarization and slowed the Aβ + IBO-induced loss of membrane potential (Fig. 1 and Tables 1 and 2). In addition, cordycepin reversed neuronal hyperactivity in the Aβ + IBO-induced evoked AP firing, including the increase in repetitive firing frequency, shortening of evoked AP latency, and reduction in the amplitude of fAHP. The decrease in the evoked AP latency/onset time accelerated the firing of APs (Fig. 3), and a lower-strength depolarizing stimulation was required to evoke AP firing (Fig. 4). A reduction in the amplitude of fAHP meant a reduction in hyperpolarizing potassium currents, which increased the overall excitability of neurons (Fig. 4) [26]. Further, pretreatment with DPCPX could block the suppressive effects of cordycepin (Figs. 1, 2, 3, 4 and Tables 1 and 2). Based on the previous findings [342728293031], it was strongly speculated that the suppressive effect of cordycepin against Aβ + IBO-induced excessive membrane depolarization and neuronal hyperactivity might be an important mechanism underlying its neuroprotective effect; cordycepin provided neuroprotection partially via the activation of A1R.
Recent studies demonstrated that cordycepin affects some cell types via the activation of four adenosine receptor subtypes (A1, A2A, A2B, and A3) to modulate a diverse range of biological activities [5]. These adenosine receptor subtypes appear in distinct distributions, and the A1 and A2A subtypes are mainly expressed in the CNS. Specifically, the A1R are widely distributed in the cortex, cerebellum, thalamus, and hippocampus [21]. Further, several studies have proved that the activation of adenosine A1R, but not A2AR, could attenuate neuronal excitability [3233]-and A1R antagonist DPCPX could reduce the ability of cordycepin to prevent glutamate-induced excitotoxicity insult in HT22 cells [5]. This accorded with the results of the present study that DPCPX could reverse the suppressive effects of cordycepin on Aβ + IBO-induced changes in the neuronal electrophysiological function. In combination with these previous studies, the results in our present study pose a strongy insight that the activation of A1R is an important mechanism involved in the neuroprotective effects of cordycepin against the Aβ + IBO-induced neuronal damage.
In conclusion, the present study provided important insights into the neuroprotective effects of cordycepin against the Aβ + IBO-induced insult through improving the neuronal electrophysiological properties. The results showed that cordycepin could significantly delay/reduce Aβ + IBO-induced excessive neuronal membrane depolarization and neuronal hyperactivity. Further, this suppressive effect could be blocked using DPCPX (an A1R-specific blocker), indicating that cordycepin might exert neuroprotective effects against Aβ + IBO-induced impairment -, and the mechanism through the activation of A1R is strong recommended, thus highlighting the therapeutic potential of cordycepin in AD.
Figures and Tables
Fig. 1
Effects of cordycepin on neuronal membrane potentials before and after drug application.
(A) A representative trace in the control group subjected to no treatment. (B) A representative trace subjected to β-amyloid (Aβ) + ibotenic acid (IBO). (C) A representative trace subjected to cordycepin (Cor) + Aβ + IBO. (D) A representative trace pretreated with DPCPX and then subjected to Cor + Aβ + IBO. (E) A representative trace pretreated with caffeine and then subjected to Cor + Aβ + IBO.

Fig. 2
Effects of cordycepin on action potential (AP) firing in primary hippocampal neurons evoked by a depolarization stimulus of 20 pA amplitude for the 600-ms duration.
(A) Sample traces showed that cordycepin reversed the increase in β-amyloid (Aβ) + ibotenic acid (IBO)-induced firing frequency. (B) Comparison of membrane potential before and after drug application. (C) Comparison of firing frequency among different drug application groups. (D) Comparison of evoked AP latency among different drug application groups. **p < 0.01, compared with the Aβ + IBO group; ##p < 0.01, compared with the cordycepin (Cor) + Aβ + IBO group.
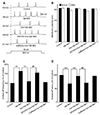
Fig. 3
Effects of cordycepin on action potential (AP) firing evoked by a depolarizing ramp stimulus in primary hippocampal neurons treated with β-amyloid (Aβ) + ibotenic acid (IBO).
(A) Sample traces of AP firing evoked by a ramp current in different groups (control, Aβ + IBO, cordycepin [Cor] + Aβ + IBO, and DPCPX + Cor + Aβ + IBO). (B) Comparison of membrane potential before and after drug application. (C) Comparison of evoked AP latency among different drug application groups. **p < 0.01, compared with the Aβ + IBO group; ##p < 0.01, compared with the Cor + Aβ + IBO group.
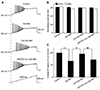
Fig. 4
Effects of cordycepin on the evoked action potential (AP) properties of primary hippocampal neurons treated with β-amyloid (Aβ) + ibotenic acid (IBO).
(A) Representative AP recording in different groups (control, Aβ + IBO, cordycepin [Cor] + Aβ + IBO, and DPCPX + Cor + Aβ + IBO). (B) AP spike width had no significant difference among different drug application groups. (C) Comparison of the fast afterhyperpolarization (fAHP) amplitude among different drug application groups. **p < 0.01, compared with the Aβ + IBO group; ##p < 0.01, compared with the Cor + Aβ + IBO group.
Table 1
Effects of cordycepin on β-amyloid (Aβ) + ibotenic acid (IBO)-induced slow membrane depolarization properties recorded in primary hippocampal neurons

ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31660275, 81660713, 31860605), Jiangxi Outstanding Youth Talent Cultivation Program (20171BCB23077), the Science and Technology Program of Department of Education of Jiangxi Province (GJJ180594, GJJ180632, GJJ170675), the Academic and Technical Leader Program of Jiangxi Province (20182BCB22005), Key Construction Laboratory Program of Jiangxi Science and Technology Normal University (2017ZDPYJD004), Special Fundation for First-class Disciplines of Chinese Medicine in Jiangxi Province (JXSYLXK-ZHYAO119).
Notes
References
1. Liu X, Hou D, Lin F, Luo J, Xie J, Wang Y, Tian Y. The role of neurovascular unit damage in the occurrence and development of Alzheimer's disease. Rev Neurosci. 2019; 30:477–484.

2. Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016; 12:459–509.
3. Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010; 9:387–398.

4. Calì T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson's disease. Biofactors. 2011; 37:228–240.

5. Jin ML, Park SY, Kim YH, Oh JI, Lee SJ, Park G. The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicology. 2014; 41:102–111.

6. Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010; 15:1382–1402.

7. Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009; 30:379–387.

8. Šišková Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T, Pitsch J, Schoch S, Becker A, von der Kammer H, Remy S. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer's disease. Neuron. 2014; 84:1023–1033.

9. Yin H, Wang H, Zhang H, Gao N, Zhang T, Yang Z. Resveratrol attenuates Aβ-induced early hippocampal neuron excitability impairment via recovery of function of potassium channels. Neurotox Res. 2017; 32:311–324.

10. Zhang J, Li P, Wang Y, Liu J, Zhang Z, Cheng W, Wang Y. Ameliorative effects of a combination of baicalin, jasminoidin and cholic acid on ibotenic acid-induced dementia model in rats. PLoS One. 2013; 8:e56658.

11. Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010; 47:264–272.
12. Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, Kleckers D, Lopez del Amo JM, Grüning BA, Wang Q, Schmidt MR, Lurz R, Anwyl R, Schnoegl S, Fändrich M, Frank RF, Reif B, Günther S, Walsh DM, Wanker EE. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat Chem Biol. 2011; 8:93–101.

13. Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1–42) and ibotenic acid. Horm Behav. 2007; 52:297–306.
14. Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Devel Ther. 2014; 8:1941–1953.
15. Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012; 47:979–987.

16. Chaicharoenaudomrung N, Jaroonwitchawan T, Noisa P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol In Vitro. 2018; 46:113–121.

17. Olatunji OJ, Feng Y, Olatunji OO, Tang J, Ouyang Z, Su Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed Pharmacother. 2016; 81:7–14.

18. Peng J, Wang P, Ge H, Qu X, Jin X. Effects of cordycepin on the microglia-overactivation-induced impairments of growth and development of hippocampal cultured neurons. PLoS One. 2015; 10:e0125902.

19. Cheng Z, He W, Zhou X, Lv Q, Xu X, Yang S, Zhao C, Guo L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol. 2011; 664:20–28.

20. Chen C, Liu XP, Jiang W, Zeng B, Meng W, Huang LP, Li YP, Sun W, Yuan CH, Yao LH. Anti-effects of cordycepin to hypoxia-induced membrane depolarization on hippocampal CA1 pyramidal neuron. Eur J Pharmacol. 2017; 796:1–6.

21. Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, Limatola C. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology. 2010; 35:1550–1559.

22. Song H, Huang LP, Li Y, Liu C, Wang S, Meng W, Wei S, Liu XP, Gong Y, Yao LH. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. Neurotoxicology. 2018; 68:73–80.

23. Ford L, Crossley M, Williams T, Thorpe JR, Serpell LC, Kemenes G. Effects of Aβ exposure on long-term associative memory and its neuronal mechanisms in a defined neuronal network. Sci Rep. 2015; 5:10614.

24. Shen H, Pan J, Pan L, Zhang N. TRPC6 inhibited NMDA current in cultured hippocampal neurons. Neuromolecular Med. 2013; 15:389–395.

25. Yao LH, Li CH, Yan WW, Huang JN, Liu WX, Xiao P. Cordycepin decreases activity of hippocampal CA1 pyramidal neuron through membrane hyperpolarization. Neurosci Lett. 2011; 503:256–260.

26. Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008; 105:15154–15159.

27. Haberman RP, Koh MT, Gallagher M. Heightened cortical excitability in aged rodents with memory impairment. Neurobiol Aging. 2017; 54:144–151.

28. Thomé A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016; 21:1257–1262.

29. Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013; 64:145–152.

30. Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010; 35:1016–1025.

31. Yuan P, Grutzendler J. Attenuation of β-Amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J Neurosci. 2016; 36:632–641.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download