Abstract
Na+/H+ exchangers (NHEs) have been shown to be involved in regulating cell volume and maintaining fluid and electrolyte homeostasis. Pooled evidences have suggested that loss of Na+/H+ exchanger isoform 8 (NHE8) impairs intestinal mucosa. Whether NHE8 participates in the pathology of infectious colitis is still unknown. Our previous study demonstrated that somatostatin (SST) could stimulate the expression of intestinal NHE8 so as to facilitate Na+ absorption under normal condition. This study further explored whether NHE8 participates in the pathological processes of infectious colitis and the effects of SST on intestinal NHE8 expression in the setting of infectious colitis. Our data showed that NHE8 expression was reduced in Citrobacter rodentium (CR) infected mice. Up-regulation of NHE8 improved diarrhea symptom and mucosal damage induced by CR. In vitro, a similar observation was also seen in Enteropathogenic E. coli (EPEC) infected Caco-2 cells. Seglitide, a SST receptor (SSTR) 2 agonist, partly reversed the inhibiting action of EPEC on NHE8 expression, but SSTR5 agonist (L-817,818) had no effect on the expression of NHE8. Moreover, SST blocked the phosphorylation of p38 in EPEC-infected Caco-2 cells. Taken together, these results suggest that enhancement of intestinal NHE8 expression by SST could ameliorate the symptoms of mice with infectious colitis.
Na+/H+ exchangers (NHEs), a family of transmembrane proteins located on the apical and basolateral epithelial membranes, display not only various tissue distributions and cellular localizations but also diverse physiological roles [1]. Of the ten mammalian NHEs isoforms, NHE1, NHE2, NHE3 and NHE8 have been identified in the intestinal epithelia. NHE1 involves in the maintenance of intracellular pH homeostasis and cell differentiation [2]. NHE2 and NHE3 are mainly localized at the apical membranes in both the intestine and kidney [3]. Compelling evidences indicated that NHE3 and NHE8 are the predominant NHEs for electrolyte/fluid absorption and secretion in intestine [34]. However, NHE2 barely compensates for the loss of NHE3 in the term of sodium absorption [5]. NHE8 is highly expressed in neonates in both the kidney and intestine when NHE3 expression is very low [67]. Xu et al. demonstrated that NHE8 plays a compensatory role for the loss of NHE activity in NHE2X3 double knockout mice [8]. Moreover, pooled evidences showed that NHE8 might contribute to gastrointestinal mucosa protection [910]. Liu et al. [11] showed that NHE8 plays a role in mucus layer formation, which is important for preventing bacteria from adhering to the epithelial surface in infectious mice. Our previous study showed that NHE8 expression was decreased in mice with Dextran Sulfate Sodium (DSS) induced colitis [12]. Of note, enhancing NHE8 level by somatostatin could ameliorate diarrhea symptoms and intestinal inflammation in DSS colitis [12]. The mechanisms that underlie the pathophysiology of diarrhea in infectious colitis are complex. It is uncovered that diarrhea is correlated with the activation of cAMP and cGMP in intestinal tract [13], and whether NHE8 plays a role in the pathology of infectious colitis has not be determined.
Somatostatin (SST) is a prominent neuropeptide that is widely distributed throughout the central nervous system and peripheral organs. Interacting with its five somatostatin receptor (SSTR) subtypes, SST functions as a neurotransmitter and hormone to produce a broad spectrum of biological responses [14]. SST and its' analogue like octreotide were used to treat secretory and functional diarrhea associated with a variety of gastrointestinal diseases, such as chemotherapy-related diarrhea [15] and refractory diarrhea [16].
In this study, we investigated the role of NHE8 expression in infectious colitis mice model for the first time. Our results showed that NHE8 expression was reduced in mice with infectious colitis. Up-regulation of NHE8 expression by SST could ameliorate the severity of infectious colitis.
Enteropathogenic E. coli (EPEC, serotype O127:K63) was obtained from the China Center of Industrial Culture Collection (Beijing, China). Citrobacter rodentium (C. rodentium, CR) was purchased from American Type Culture Collection (Manassas, VA, America). Bacteria were cultured in LB broth at 37℃ overnight. For the CR strain, 2.5×108 CFU/ml of bacteria were resuspended in in phosphate-buffered saline (PBS) before use. For the EPEC strain, 1×108 CFU/ml of bacteria were resuspended in MEM-NEAA medium before use [17].
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA, America) and cultivated in MEM medium (Hyclone, Thermal scientific; MA, America), supplemented with 10% fetal bovine serum (FBS) (Gibco, life technologies; Carlsbad, CA, America), 1% penicillin/streptomycin and 1% non-essential amino acids (Hyclone, Thermal scientific; MA, America). For the bacterial infection study, Caco-2 cells were used at 80–90% confluence. Cells were exposed to 1×108 CFU EPEC in 10 ml of antibiotic-free MEM-NEAA medium for 1 h, and then treated with SST (1 µM; Sigma-Aldrich, MO, America),SST receptor (SSTR) 2 agonist seglitide (1 µM; TOCRIS Bioscience, Ellisville, MO, America), or SSTR5 agonist L-817,818 (500 nM; TOCRIS Bioscience, Ellisville, MO, America), respectively. After washing three times with PBS to remove unattached bacterial, Caco-2 cells were harvested.
Six to eight week old wide-type C57BL/6 mice were obtained from the Animal Experiment Center of West China Hospital (Sichuan, China). Mice were housed with adlibitum access to rodent food and water. In animal experiments, SST is limited due to its short half-life (a few minutes). For this reason, we used its analogue octreotide (Novartis Pharma Stein AG, Switzerland) which has a longer half-life (60–90 min). Mice were randomly divided into three groups: control, C. rodentium treated (CR), C. rodentium+octreotide treated (CR+Oct) groups (9–10 mice per group). Control mice were established by 200 µl LB medium gavage. CR and CR+Oct groups were established by 200 µl LB medium gavage for twelve consecutive days, and then were subcutaneous administered with saline for CR group or octreotide for CR+Oct group for three days (50 µg/kg body weight, three times per day). Mice were euthanized on the 15th day after the first gavage with CR or LB. Colonic tissues were collected for hematoxylin-eosin (H&E) and western blot detection. All animal study procedures were performed after receiving the approval of the Institutional Animal Care and Use Committee in Sichuan University (IACUC approval No. 2015006A).
Severity of colitis was assessed by diarrhea scores based on the shape, color and hardness of the fecal material. A score of 1 represents normal feces, a score of 2 represents exceptionally loose feces, a score of 3 represents loose yellow-green feces, and watery feces were given a score of 4. A score greater than 2 was considered to be diarrhea, as described previously [18].
To detect the concentrations of SST in the colonic tissue, all tissues were homogenized, then kept at −20℃ until the radioimmunoassay (RIA) method was performed. The detection procedures were carried out in Beijing DORUN international Technology Co. Ltd. (Beijing, China).
The middle part of the colon was removed and fixed in 4% phosphate-buffered formalin, embedded in paraffin, sectioned, and the sections were stained with hematoxylin-eosin (H&E). Histological analysis of colitis severity was assessed for aberrant crypt architecture, epithelial hyperplasia, epithelial damage and inflammatory cell infiltration, as described previously [19].
Colonic tissue and Caco-2 cell lysates were prepared in RIPA lyses buffer (Beyotime; Beijing, China). Protein concentration was measured by the Bradford method according to the manufactures' instruction (Beyotime; Beijing, China). Samples were separated on 8% SDS-polyacrylamide gel and transferred to PVDF membranes. Membranes were blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T), followed by an incubation with primary antibodies: anti-NHE8 (kindly provided by Ghishan's lab, University of Arizona Health Science Center, Tucson, America), anti-pp38, anti-p38, anti-pERK, anti-ERK (R&D System, Minneapolis, America) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Goodhere, Hangzhou, China). Then, signals from immunoreactive complexes were detected with the BM Chemiluminescence western blotting kit (Roche Diagnostics; Indianapolis, IN, America). The ratio of NHE8 protein intensity to GAPDH protein intensity was used for protein expression quantitation.
Statistics were performed using SPSS 13.0 software. Data are presented as the means±standard deviation. For experimental data between two groups, unpaired Student's t-test was used. For experimental data among multiple groups, a one-way ANOVA was used. Differences were considered statistically significant when the p value was less than 0.05.
The CR infected-mice are used to model diarrheal diseases in humans. Mice in the control group did not develop diarrhea at any time point. In CR-infected mice, the onset of diarrhea occurred at the third day post-infection with a diarrhea score of 2.33±0.52, and continually exacerbated throughout the experiment (Fig. 1A). The CR-infected mice displayed an apparent trend of lower SST content compared to control mice (3.52±0.40 pg/ml in control group vs. 1.19±0.20 pg/ml in CR group; p<0.05) (Fig. 1B). Histological observation indicated that the architecture of the epithelial layer was disrupted in the colon specimens after the CR infection (Fig. 1C).
To elucidate the role of NHE8 in infectious colitis, we analyzed NHE8 protein abundance by immunoblot analysis. As shown in Figs. 2A and 2B, CR-infected mice exhibited a significant decrease in intestinal NHE8 protein expression compared with control mice (0.59±0.05 in CR group vs. 0.94±0.20 in control group in the proximal colon; 0.26±0.06 in CR group vs. 0.56±0.13 in control group in the distal colon; p<0.05). Similarly, EPEC infection significantly reduced the NHE8 protein expression in Caco-2 cells (0.79±0.32 in EPEC-infected group vs. 1.57±0.10 in control group; p<0.05; Fig. 2C).
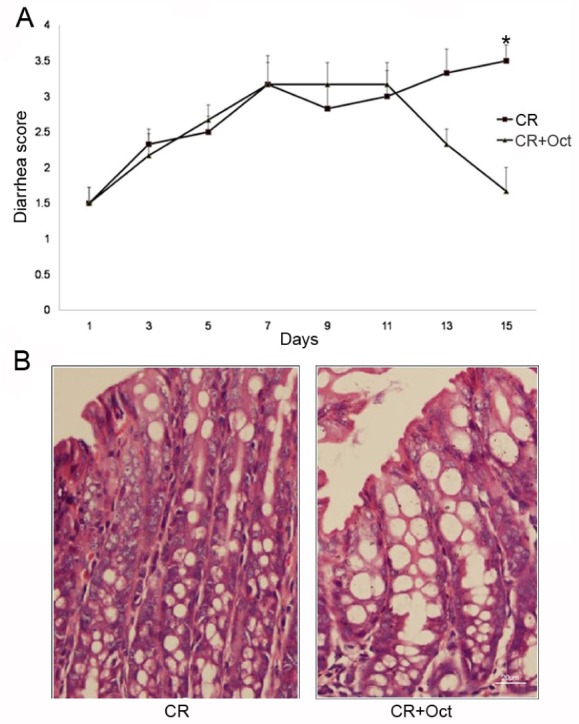
After administrating with octreotide, a significant improvement in the diarrhea score was observed in octreotide treated CR-infected mice compared with CR-infected mice (1.67±0.82 in CR+Oct group vs. 3.50±0.55 in CR group; p<0.05) (Fig. 3A), the epithelial layer structure was protected as well (Fig. 3B). At the same time, the NHE8 protein expression was significantly increased in octreotide treated CR-infected mice compared with CR-infected mice (1.10±0.20 in CR+Oct group vs. 0.59±0.05 in CR group in the proximal colon; 0.78±0.25 in CR+Oct group vs. 0.26±0.06 in CR group in the distal colon; p<0.05; Figs. 2A and 2B).
To elucidate how SST regulated NHE8 in infectious colitis, we further examined the possible mechanisms in Caco-2 cells. Similarly, our data showed that SST could stimulate the NHE8 protein expression in EPEC-infected Caco-2 cells (1.15±0.22 in SST-treated group vs. 0.79±0.32 in EPEC-infected group; p<0.05; Fig. 2C).
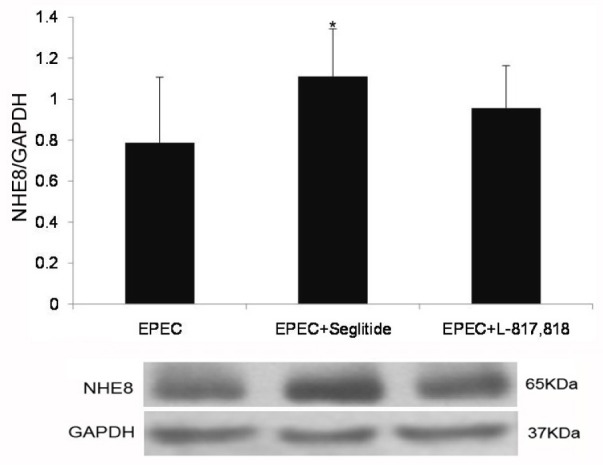
Previous studies identified that SSTR2 and (or) SSTR5 are involved in SST-mediated NHE8 stimulation [2021]. Thus, we further explored the role of SSTR2 and SSTR5 in SST-mediated NHE8 expression in EPEC-infected Caco-2 cells. As shown in Fig. 4, seglitide significantly up-regulated the expression of NHE8 protein in EPEC-infected Caco-2 cells (0.79±0.32 in EPECinfected group vs. 1.11±0.23 in seglitide-treated group; p<0.05). However, L-817,818 had no effect on the NHE8 protein expression in EPEC-infected cells (0.79±0.32 in EPEC-infected group vs. 0.96±0.21 in L-817,818-treated group; p>0.05).
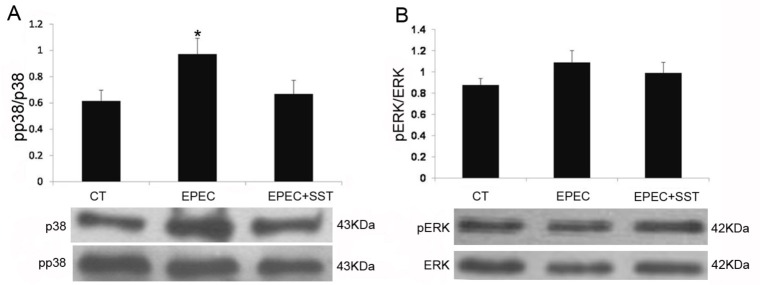
A previous study elucidated that SST regulates NHE8 expression via the SSTR2-p38 mitogen-activated protein kinase (MAPK) pathway under normal physiological conditions [20]. Therefore, a western blot was performed to determine the involvement of the MAPK pathway in EPEC-infected Caco-2 cells. As shown in Fig. 5A, compared with control, EPEC significant activated the phosphorylation of p38 (0.62±0.08 in control group vs. 0.97±0.12 in EPEC-infected group; p<0.05). The phosphorylation of p38 was significantly reduced in SST-treated cells compared with EPECinfected cells (0.67±0.10 in SST-treated group vs. 0.97±0.12 in EPEC-infected group; p<0.05). However, the status of phospho-ERK1/2 did not changed in EPEC-infected group compared with control group (p>0.05, Fig. 5B).
Many enteric pathogens like EPEC are known to induce diarrhea through altering electrolyte transport, which including the suppression of intestinal Na+ absorption [22]. Of note, NHEs are implicated in sodium absorption, mucosal protection and inflammatory response. For example, NHE8 plays a role in maintaining the integrity of the gastrointestinal mucosa [91011]. In fact, previous studies have suggested that NHEs directly contributed to EPEC-associated diarrhea [23]. Thus, facilitating the expression of NHEs might have beneficial effect on the management of infectious colitis. Our previous study clearly revealed that SST could stimulate gastrointestinal sodium absorption by increasing NHE8 expression but not NHE3 expression under normal physiological conditions [20]. Accordingly, we further examined the exact role of intestinal NHE8 in infectious colitis, and the effects of SST by modulating NHE8 in response to infectious colitis.
CR shares similar virulence factors with EPEC. Therefore, the CR infection in mice has been used as a model to study the molecular basis of EPEC in vivo [24]. Observations including mild diarrhea and body weight loss demonstrated that mice developed colitis by CR gavage. Moreover, the CR induced colitis mice exhibit a loss of colonic NHE8. A previous study documented that the number of SST-positive enteroendocrine cells was significantly decreased following a CR infection [25], and our data also suggested that a decreased SST content was observed in infectious colitis mice compared with control mice. Of note, supplement of octreotide, a SST analog, could effectively increase the expression of NHE8 in CR infected mice. Meanwhile, the severity of infectious colitis was significantly ameliorated after up-regulating colonic NHE8 expression by octreotide. Therefore, facilitating colonic NHE8 expression is beneficial to restore intestinal mucosa integrity, which in accordance with previous studies [2627].
Caco-2 cells infected with EPEC were applied to further explore the mechanisms of this protective effect. Previous study suggested that EPEC exerts a differential response on the activity of NHE isoform, with a significant activation of NHE2 and a marked inhibition of NHE3 activity [23]. However, no information about the effect of EPEC on NHE8 isoform has been discussed. The present study showed that an infection of Caco-2 cells with EPEC resulted in a significant inhibition of NHE8 expression. Treated with SST, EPEC-infected Caco-2 cells could restore the NHE8 expression. The concentration of SST used in our study was in accordance with previous studies, which have shown that SST at the physiological range (1–100 nM) profoundly blocked intestinal electrolyte secretion in vivo and in vitro [2829].
SST induces its biological effects through binding to SSTRs. SSTR subtypes expressed in Caco-2 monolayers still have discrepancies among different studies [3031]. Our previous study showed that SSTR 1, 2, 4 and 5 were expressed in Caco-2 cells [20]. The difference among these studies may be attributed to the heterogeneity of the Caco-2 cell lines. Of note, previously published studies demonstrated that in rat colonic epithelium, SSTR2 and 5 mediated SST exert anti-secretory effects [3233]. Thus, the involvement of SSTR2 and 5 were further examined in SST-mediated NHE8 up-regulation. Our data indicated that SSTR2 agonist seglitide, but not SSTR5 agonist L-817,818, increased the NHE8 level in Caco-2 cells. Therefore, SSTR2 activation by SST plays an important role in SST-stimulated NHE8 expression in the Caco-2 monolayers infected with EPEC.
Moreover, it is well established that inflammatory stimuli could trigger major intracellular signaling pathways, such as the MAPK pathway, in particular p38 MAPK [34]. MAPKs which comprise ERK, p38, and JNK have been known to be involved in cell proliferation, cell survival and inflammation [35]. SST could trigger a range of intracellular pathways including sustained activation of MAPKs through coupling to its receptors. Of note, activated SSTR2 either stimulates or inhibits the MAPK activity, whereas activated SSTR5 could inhibit MAPK activity [36]. Our data indicated that SST could inhibit p38 phosphorylation in EPEC-infected caco-2 cells, while no apparent change was observed with respect to the levels of phospho-ERK. Intriguingly, SST could stimulate intestinal NHE8 expression through activation of the p38 MAPK pathway under normal physiological conditions [20]. One possible reason for discrepant results is that SST might directly modulate the p38 MAPK signal pathway in a different way in response to the cellular environment and stimuli. Another possible explanation is that SST suppressed EPEC induced activation of p38 phosphorylation, on the consideration that EPEC has been shown to activate the p38 MAPK pathway [37].
In conclusion, the present study demonstrated that SST could stimulate NHE8 expression in infectious colitis, which results in improving diarrheal symptoms and mucosal structure. Furthermore, SST exerts its anti-diarrheal effects by stimulating NHE8 expression through activation of SSTR2. Moreover, further study need to determine the downstream signal transduction pathway involved in SST-mediated NHE8 stimulation.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Fund of China grant 81070294.
Notes
References
1. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005; 67:411–443. PMID: 15709964.
2. Fliegel L. The Na+/H+ exchanger isoform 1. Int J Biochem Cell Biol. 2005; 37:33–37. PMID: 15381146.
3. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998; 19:282–285. PMID: 9662405.
4. Aronson PS. Ion exchangers mediating NaCl transport in the renal proximal tubule. Cell Biochem Biophys. 2002; 36:147–153. PMID: 12139400.

5. Woo AL, Noonan WT, Schultheis PJ, Neumann JC, Manning PA, Lorenz JN, Shull GE. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol. 2003; 284:F1190–F1198. PMID: 12582007.
6. Baum M, Martin MG, Booth IW, Holmberg C, Twombley K, Zhang Q, Gattineni J, Moe O. Nucleotide sequence of the Na+/H+ exchanger-8 in patients with congenital sodium diarrhea. J Pediatr Gastroenterol Nutr. 2011; 53:474–477. PMID: 21666503.
7. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol. 2005; 289:G36–G41. PMID: 15731506.

8. Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G647–G653. PMID: 21252044.

9. Wang A, Li J, Zhao Y, Johansson ME, Xu H, Ghishan FK. Loss of NHE8 expression impairs intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2015; 309:G855–G864. PMID: 26505975.

10. Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol. 2013; 304:G257–G261. PMID: 23220221.

11. Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol. 2013; 305:C121–C128. PMID: 23657568.

12. Li X, Cai L, Xu H, Geng C, Lu J, Tao L, Sun D, Ghishan FK, Wang C. Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G954–G963. PMID: 27686614.

13. Payne CM, Fass R, Bernstein H, Giron J, Bernstein C, Dvorak K, Garewal H. Pathogenesis of diarrhea in the adult: diagnostic challenges and life-threatening conditions. Eur J Gastroenterol Hepatol. 2006; 18:1047–1051. PMID: 16957509.

14. Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J Mol Endocrinol. 2014; 52:R223–R240. PMID: 24647046.

15. Pai V, Porter K, Ranalli M. Octreotide acetate is efficacious and safe in children for treating diarrhea due to chemotherapy but not acute graft versus host disease. Pediatr Blood Cancer. 2011; 56:45–49. PMID: 21108438.

16. Szilagyi A, Shrier I. Systematic review: the use of somatostatin or octreotide in refractory diarrhoea. Aliment Pharmacol Ther. 2001; 15:1889–1897. PMID: 11736719.

17. Li X, Wang Q, Xu H, Tao L, Lu J, Cai L, Wang C. Somatostatin regulates tight junction proteins expression in colitis mice. Int J Clin Exp Pathol. 2014; 7:2153–2162. PMID: 24966923.
18. Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J, Einerhand AW. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol. 2003; 77:13005–13016. PMID: 14645557.
19. Hainzl E, Stockinger S, Rauch I, Heider S, Berry D, Lassnig C, Schwab C, Rosebrock F, Milinovich G, Schlederer M, Wagner M, Schleper C, Loy A, Urich T, Kenner L, Han X, Decker T, Strobl B, Müller M. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J Immunol. 2015; 195:5011–5024. PMID: 26432894.

20. Wang C, Xu H, Chen H, Li J, Zhang B, Tang C, Ghishan FK. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol. 2011; 300:C375–C382. PMID: 21106692.

21. Li X, Cai L, Xu H, Geng C, Lu J, Tao L, Sun D, Ghishan FK, Wang C. Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G954–G963. PMID: 27686614.

22. Sandle GI. Infective and inflammatory diarrhoea: mechanisms and opportunities for novel therapies. Curr Opin Pharmacol. 2011; 11:634–639. PMID: 21983454.

23. Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G370–G378. PMID: 15075254.
24. Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014; 12:612–623. PMID: 25088150.

25. O'Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol. 2006; 8:646–660. PMID: 16548890.
26. Eliakim R, Karmeli F, Okon E, Rachmilewitz D. Octreotide effectively decreases mucosal damage in experimental colitis. Gut. 1993; 34:264–269. PMID: 8381760.

27. van Bergeijk JD, Wilson JH. Somatostatin in inflammatory bowel disease. Mediators Inflamm. 1997; 6:303–309. PMID: 18472863.

28. McKeen ES, Feniuk W, Humphrey PP. Somatostatin receptors mediating inhibition of basal and stimulated electrogenic ion transport in rat isolated distal colonic mucosa. Naunyn Schmiedebergs Arch Pharmacol. 1995; 352:402–411. PMID: 8532068.

29. Warhurst G, Barbezat GO, Higgs NB, Reyl-Desmars F, Lewin MJ, Coy DH, Ross I, Grigor MR. Expression of somatostatin receptor genes and their role in inhibiting Cl-secretion in HT-29cl.19A colonocytes. Am J Physiol. 1995; 269:G729–G736. PMID: 7491965.
30. Ayiomamitis GD, Notas G, Zaravinos A, Drygiannakis I, Georgiadou M, Sfakianaki O, Mastrodimou N, Thermos K, Kouroumalis E. Effects of octreotide and insulin on colon cancer cellular proliferation and correlation with hTERT activity. Oncoscience. 2014; 1:457–467. PMID: 25594044.

31. Saksena S, Theegala S, Bansal N, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2009; 297:G878–G885. PMID: 20501436.

32. Hope N, Butt G, Ross I, Warhurst G, Arn M, Grigor M, Lubcke R, Barbezat GO. Somatostatin enhances cAMP-dependent shortcircuit current in human colon via somatostatin receptor subtype-2. Dig Dis Sci. 2001; 46:2499–2503. PMID: 11713960.
33. Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology. 1996; 111:325–333. PMID: 8690197.

34. O'Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006; 5:549–563. PMID: 16773072.
35. Chang JP, Habibi HR, Yu Y, Moussavi M, Grey CL, Pemberton JG. Calcium and other signalling pathways in neuroendocrine regulation of somatotroph functions. Cell Calcium. 2012; 51:240–252. PMID: 22137240.

36. Ben-Shlomo A, Pichurin O, Barshop NJ, Wawrowsky KA, Taylor J, Culler MD, Chesnokova V, Liu NA, Melmed S. Selective regulation of somatostatin receptor subtype signaling: evidence for constitutive receptor activation. Mol Endocrinol. 2007; 21:2565–2578. PMID: 17609435.

37. Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect Immun. 2001; 69:1298–1305. PMID: 11179291.
Fig. 1
Establishment of infectious colitis mice models by C. rodentium(CR) gavage.
Various parameters were observed. (A) Diarrhea scores; (B) Concentration of somatostatin (SST) in CR-infected mice. (p<0.05 for CR group vs. CT (control group); unpaired Student's t-test). (C) colonic histology observation (×400 magnification). Results were reported as the mean±SD from 18 mice (p<0.05 for CR [n=9] vs. CT [n=9]; unpaired Student's t-test).
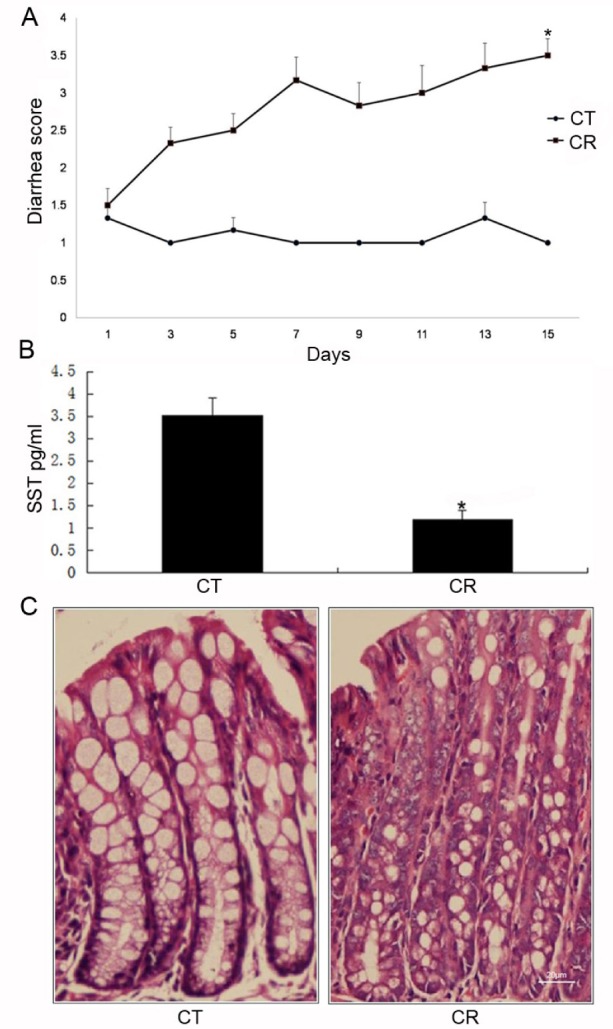
Fig. 2
Expression of Na+/H+ exchanger 8 (NHE8) protein in C. rodentium-infected mice and EPEC-infected Caco-2 cells.
NHE8 expression was calculated by the band density of the NHE8 normalized to the GAPDH band. Results are presented as the mean±SD from 3 independent experiments. (A) Expression of NHE8 protein in the proximal colon. (B) Expression of the NHE8 protein in the distal colon (p<0.05 for C. rodentium-infected mice (CR) vs. control mice (CT) or octreotide-treated CR-infected mice (CR+Oct); one-way ANOVA). (C) Expression of NHE8 protein in EPEC-infected (EPEC) Caco-2 cells (p<0.05 for EPEC group vs. CT or SST-treated group [EPEC+SST]; one-way ANOVA).
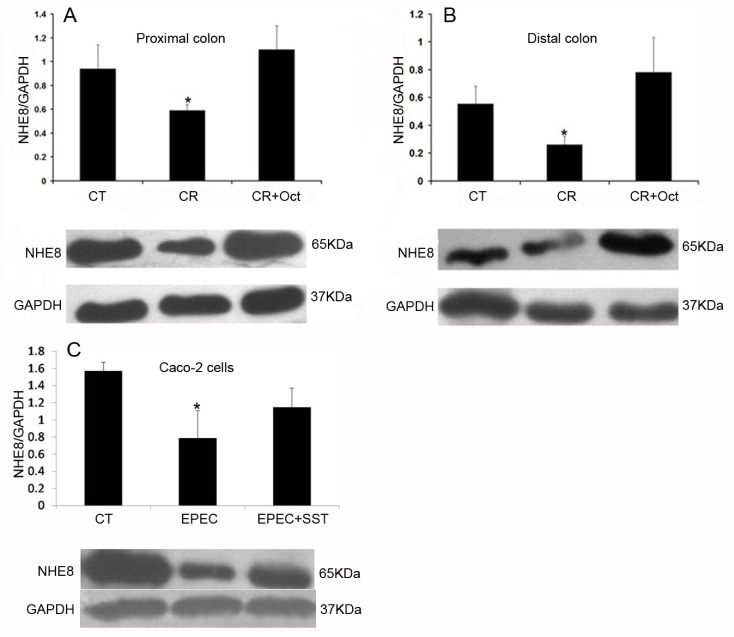
Fig. 3
(A) Effect of octreotide on diarrhea score in C. rodentium-infected mice (p<0.05 for CR vs. CR+Oct; unpaired Student's t-test). (B) Colonic histology observation in both two groups (×400 magnification).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download