Abstract
The aim of this study was to determine the raising anticancer effects of resveratrol (Res) on paclitaxel (PA) in non-small cell lung cancer (NSCLC) cell line A549. The 10 µg/ml of Res had no effect on human fetal lung fibroblast MRC-5 cells or on A549 cancer cells and the 5 or 10 µg/ml of PA also had no effect on MRC-5 normal cells. PA-L (5 µg/ml) and PA-H (10 µg/ml) had the growth inhibitory effects in NSCLC cell line A549, and Res increased these growth inhibitory effects. By flow cytometry experiment, after Res (5 µg/ml)+PA-H (10 µg/ml) treatment, the A549 cells showed the most apoptosic cells compared to other group treatments, and after additional treatment with Res, the apoptosic cells of both two PA concentrations were raised. Res+PA could reduce the mRNA and protein expressions of COX-2, and Res+PA could reduce the COX-2 related genes of VEGF, MMP-1, MMP-2, MMP-9, NF-κB, Bcl-2, Bcl-xL, procollagen I, collagen I, collagen III and CTGF, TNF-α, IL-1β, iNOS and raise the TIMP-1, TIMP-2, TIMP-3, IκB-α, p53, p21, caspase-3, caspase-8, caspase-9, Bax genes compared to the control cells and the PA treated cells. From these results, it can be suggested that Res could raise the anticancer effects of PA in A549 cells, thus Res might be used as a good sensitizing agent for PA.
Cancer is the second most serious disease that threatens human health; therefore, it is a big dream of scientists to conquer cancer. Natural product is one of the most important way from which new drug and lead compound may possibly be discovered and from which actually directly or indirectly come many kinds of anticancer drugs. Paclitaxel is now the most outstanding natural anticancer drug among those discovered which is used widely in clinical practice in treatment of breast cancer, ovarian cancer, lung cancer and some of head and neck cancer [12].
Paclitaxel may make tubulin and tubulin dimer comprising microtubule lose dynamic balance, induce and promote polymerization of tubulin, microtubule assembly and prevent depolymerization, then enabling microtubule to be stable, inhibiting mitosis of cancer cells and avoiding induced apoptosis, and further effectively preventing proliferation of cancer cells and then realizing the effect of anticancer [3]. The director of National Cancer Institute (America), Dr. Broder once indicated that: Paclitaxel in the train of doxorubicin and cisplatin is the drug with best curative effect and least adverse effect in human struggle against various types of cancers, which is qualified to be called as “the last defender against terminal cancer” [4]. At present, there has been great progress in the research on obtaining paclitaxel through biosynthesis, fungal fermentation, plant tissue and cell culture and other technical methods [5].
Resveratrol is a non-flavanoids polyphenolic compound containing stilbene structure, which, according to relevant research, has various biological and pharmacological actions favorable to human health, in addition to its enhancement effect to disease resistance in plant. Resveratrol owns many kinds of biological actions such as antineoplastic activity, protective effect concerning cardiovascular system, antioxidant and antimicrobial activity. Among the pharmacologic actions of resveratrol, the most remarkable one is its role in anticancer [67]. Jang, among others, is one of the first discoverers who have found that resveratrol has anticancer activity. Further studies found that resveratrol may restrain or even reverse the three stages, i.e. initiation, proliferation and occurrence, of cancer [8]. Meanwhile, study has shown that, the combination of resveratrol and natural active substance is more effective in scavenging ABTS+free radical compared to a single component [9]. Recent studies show that resveratrol can also improve the sensitivity of chemotherapeutic drugs and apoptosis induced by radiation, as well as promote the radiation sensitivity of lung cancer A549 cell through inhibiting the expression of apoptin survivin and facilitating apoptosis [10].
COX-2 is an inducible COX that cannot be detected in most tissues under normal physiological condition; the expression of cells will increase when they are stimulated by inflammatory stimulus, certain cytokines and tumor promoter [11]. In recent years, researches show that COX-2, besides its effect on inflammatory reaction, is also closely associated with cancer. Many researches indicate that regulation and control of COX-2 and its related expression can control the growth of cancer cells and then effectively restrain cancer [12]. In this study, resveratrol raises anticancer effects of paclitaxel through COX-2 expression were determined by in vitro experiments.
NSCLC cell line A549 and human fetal lung fibroblast MRC-5 cells were purchased from Conservation Genetics CAS Kunming Cell Bank (Kunming, Guangxi, China). These cells were sustained into RPMI1640 medium (Gibco BRL, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco Co., Birmingham, MI, USA) and cultivated in culture flasks under a humidified atmosphere containing 5% CO2 at 37℃. The medium was changed twice a week.
A549 cells being in the logarithmic phase were selected, respectively inoculated into a 96-well (100 µl/well) 100 microwell plate in the size of 4×104 cells/ml and cultured overnight until occurrence of cell adherence. Different concentrations of Res (resveratrol) and PA (paclitaxel) were added into corresponding test well and then culture process continued for another 72 h; then culture medium was removed, and 100 µl nutrient solution containing 0.5 mg/ml MTT was added, then the culture process continued again for another 4 h. Afterwards, MTT was absorbed, into which 100 µl DMSO was added to make the MTT crystallized and completely dissolved, finally, OD value under the wave length of 490 nm was detected by micro-plate reader and the ratio of suppression by drugs on cells was thereby calculated [13].
In A549 cells being in the logarithmic phase, the Res and PA were added, and then sequentially cultured for in an incubator for 24 h; thereafter, supernatant and adherent cells were collected. Cell concentration was regulated to 1×106/ml and was stained by using Apoptosis Detection kit and then flow cytometry was used to detect cells. Such detection kit detected apoptotic cells through the combination of Annexin V with exposed phosphatidylserine and PI with cell nucleus.
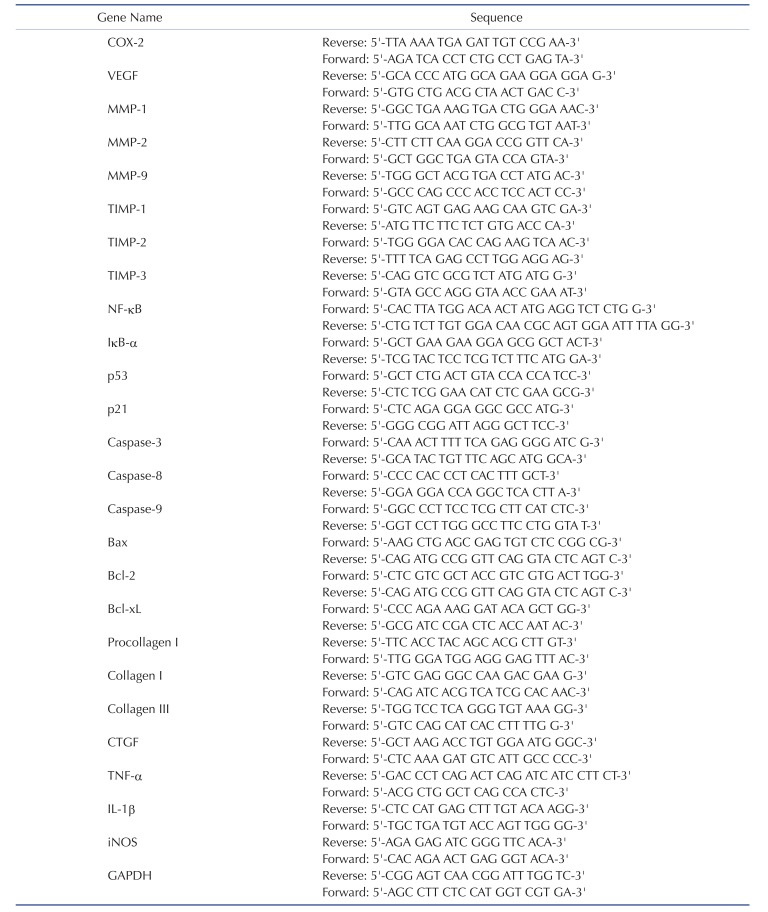
Total cell RNAs were extracted from A549 cells in logarithmic phase according to the instruction of Trizol one-step method regarding mRNA and their purity and concentration were measured. Synthesis of cDNA followed the instruction of TaKaRa Reverse Transcription System. GAPDH was used as the internal reference; primers were synthesized by Beijing Genomics Institute with the primer sequence detailed in Table 1. In line with the instruction of TaKaRa System, the 10 µl reaction system was selected, including SYBRPremix ExTaq II (2X) 5 µl, cDNA template 0.8 µl, upstream and downstream primers (10 µmol · L-1) each of 0.4 µl, dH2O 3.4 µl. Amplification condition: pre-heat for 30s under 95℃; degeneration for 5s under 95℃, annealing temperature for 30s and amplification for 39 circles; finally, extension for 5 s under 65℃; each group with three parallel holes [14]. A group's relative expression level was the ratio of gene expression quantity of such group to the internal reference GAPDH.
Protein was extracted after collecting cell lysis by utilizing A549 cells being in the logarithmic phase. Protein content in cell lysates was measured with the BCA method, and equal-quantity protein was isolated with the 12% SDS-PAGE method and transferred to PVDF membrane and then incubated for 4 h under indoor temperature by applicable monoclonal antibody to detect target protein. Primary antibody was washed away and another 2 h incubation followed with second antibody connected with HRP under indoor temperature; then after lavation, immune response band was showed on ECL blotting kit [13]. β-actin was the internal reference.
The 0~10 µg/ml of Res or PA treatment could not inhibit the growth of MRC-5 normal cells (Fig. 1A, B). The 0~10 µg/ml of Res also could not inhibit the growth of A549 cancer cells, but PA inhibiting the growth of A549 cells depended on the concentration of PA. After these experiment, the 10 µg/ml of Res, 5 and 10 µg/ml of PA were chosen for further experiment.
After treated with Res and PA, the growth inhibitory effects were determined, the control cells had the highest OD540 value (Table 2), PA could inhibit the growth of A549 cells, and the adding of Res could raise the inhibitory effects of PA treatment.
The control cells had 2.4±0.2% apoptotic cells (sub-G1 DNA content), after PA or Res+PA treatment, the apoptotic cells were increased (Fig. 1C). The PA-L (5 µg/ml), Res (10 µg/ml)+PA-L, PA-H (10 µg/ml) and Res+PA-H had the 12.6±1.2%, 19.1±1.1%, 25.6±2.1% and 33.6±2.4% apoptotic cells.
By Real-Time PCR assay and western blot experiment, the untreated A549 cells showed the strongest COX-2 and VEGF mRNA and protein expression (Fig. 2A). The COX-2 mRNA expressions of PA-L, Res+PA-L, PA-H, Res+PA-H were 0.82, 0.71, 0.57, 0.36 folds of control cells, and PA-L, Res+PA-L, PA-H, Res +PA-H also reduced the COX-2 protein expressions compared to the control cells. Res+PA-H treated A549 cells had the lowest VEGF mRNA and protein expressions.
PA could decrease the MMP-2, MMP-9 mRNA and protein expressions and increase TIMP-1, TIMP-2 mRNA expressions compared to the control A549 cells (Fig. 2B), and adding Res to PAtreatment, could enhance these decreasing and increasing effects, also the higher the PA concentration the stronger the activity compared to low concentration.
PA-L, Res+PA-L, PA-H and Res+PA-H could decrease the MMP-1 mRNA expression to 77%, 53%, 34% and 19% of control cells (Fig. 2C), meanwhile, increase these expression to 201%, 235%, 287% and 329% of control cells.
The Res+PA-H treated cells had the lowest NF-κB and highest IκB-α mRNA and protein expression (Fig. 3A), these NF-κB expressions were lower than when treated only with PA-H and IκB-α expressions also were stronger when treated only with PA-H t. In the low PA treated A549 cells, NF-κB and IκB-α expressions were similar to the tendencies of PA-H treatment.
The control cells had the weakest p53 and p21 mRNA and protein expressions (Fig. 3B), PA raised p53 and p21 expressions compared to the control cells, and the higher the PA concentration the stronger the expressions compared to low PA concentration. Res enhanced p53 and p21 mRNA and protein expressions of PA treated cells.
PA-H treated cells had stronger caspase-3, caspase-8 and caspase-9 mRNA and protein expressions than PA-H, Res+PA-L treated cells and control cells (Fig. 3C). After adding Res treatment, the caspase-3, caspase-8 and caspase-9 expressions in PA-H treated A549 cells were raised.
Res+PA-H treated A549 cells had the strongest Bax mRNA and protein expressions and weakest Bcl-2 and Bcl-xL expressions (Fig. 3D). Res+PA-L also could raise Bax expressions and reduce Bcl-2, Bcl-xL expressions compared to the only PA-L treated cells and control cells, the Bax expressions were lower and Bcl-2, Bcl-xL were higher than PA-H treatment.
PA reduced the procollagen I, collagen I, collagen III and CTGF mRNA expression compared to the control cells (Fig. 4A), and the higher concentration of PA could reduce more procollagen I, collagen I, collagen III and CTGF mRNA expression. Res could help the PA decrease procollagen I, collagen I, collagen III and CTGF expression, these expressions of Res+PA-H treated A549 cells were weakest.
TNF-α, IL-1β and iNOS mRNA expression of Res+PA-H treated A549 cancer cells were lowest, these expressions were only 23%, 25% and 13% (Fig. 4B).
After celecoxib (15 µg/ml) and Res+PA-H treated NSCLC cell line A549, the COX-2 expression in celecoxib and Res+PA-H treated cells were similar on the whole (Fig. 4C), the expressions were 0.31±0.04 and 0.33±0.03 of control cells.
COX-2 is a important factor of cancer, animal experiments manifest that expression of COX-2 can be detected in early canceration and grows with the development of carcinogenesis, which is more evident in late-stage liver cancer; and COX-2 is considered to have an important role in the occurrence and development of liver cancer [15]. Clinical outcomes reveal that the cancer tissue of poorly differentiated tissue has stronger staining than the non-cancer tissue next to it and the expression rate of COX-2 in highly differentiated cancer tissue is obviously greater that in moderately differentiated and pooly differentiated liver cancer tissue [16]. In addition to the expression of COX-2 in cancer cells, there is also excessive expression of COX-2 in new vessels around tumor; thus, excessive expression of COX-2 goes hand in hand with the generation of new vessels of tumor. Generation of tumor vessels is a condition precedent to the growth of tumor and is related to the stimulation of many growth factors, among which VEGF has the strongest effect [17]. COX-2 may play a regulatory role in the secretion of VEGF and a promoting role in tumor vessels and affects vasifaction of HCC through regulating the expression of VEGF. The extent to which VEGF may express has indivisible relationship with the MVD value, which hints that VEGF is able to stimulate the growth, infiltration and migration of HCC by enhancing the production of tumor vessels and influence patients' prognosis [18]. These studies show that COX-2 has the effect on promoting the creation of new vessels of tumor.
The researches regarding the proliferation of cancer cells and influences of expression of MMP-2 and TIMP-2 mRNA reveal that NS-398 restrain significantly the expression of MMP-2 mRNA in the liver cancer cells induced by HGF, therefore enabling the ratio of TIMP-2 mRNA to MMP-2 mRNA to increase [19]. Consequently, the selective COX-2 inhibitor NS-398 could reduce and curb the activity of MMP-2 by lowering MMP-2 and increasing the expression of TIMP-1 and the ratio of TIMP-2 mRNA/MMP-2 mRNA, thus achieving the control of migration of liver cancer cells [20]. In addition, in animal experiments, the utilization of selective inhibitor NS-398 of COX-2 has the capacity of repressing growth of liver cancer cells, facilitating death of liver cancer cells, and reducing the expression extent of VEGF mRNA and blood vessel density of liver cancer at the same time. Observation of the effect of aspirin and selective inhibitor NS-398 of COX-2 on the invasive ability of liver cancer cells regulated by HGF show that: HGF may accelerate migration of liver cancer cells and improve its invasive ability as well as increase the expression of MMP-9, then realizing the avoidance of cancer cells; NS-398 inhibits the activity of the ERK-1/2 enzyme induced by HGF and the phosphorylation of the subordinate two important components, i.e. RSK and Elk-1 of such enzyme, and therefore, cause the transcriptional activity of the transcription factor Elk-1, NF-κB and AP-1 to be prevented, which directly impact the gene regulation of MMP-9; and this suggests that COX-2 inhibitor reduces the invasive ability of liver cancer induced by HGF by the way of MAPK [2122]. MMP-1 was showed in a low level in many kinds of cancer, such as lung cancer, colon cancer, bladder cancer, breast cancer, hepatocellular carcinoma, pancreatic cancer [23]. TIMP-3 could inhibit the activities of MMP-1, MMP-2, MMP-3 and MMP-9, high TIMP-3 level could inhibit the cancer [24]. TIMP-1/2 inhibits the degradation of ECM by inhibiting the activation of MMP-2/9, which plays an important role in the formation of pulmonary fibrosis, pulmonary fibrosis may develop into lung cancer [25].
Further studies indicate that induction of the death of A549 cell may be realized by virtue of the inhibition of transcription of COX-2 by inhibiting NF-κB activity, on the one hand, and the prevention against NF-κB entering into cell and activating transcription of COX-2 by control of dissociation of IκB and NF-κB which is achieved through inhibition of phosphorylation of IkB, on the other hand [26]. The excessive expression of COX-2 can lower the downstream target genes of p53, therefore, apoptosis induced by p53 may be prevented; additionally, p21 is the CDKI downstream p53 gene; normally, the quantity of p21 is extremely few, and p21 will play a role of tumor suppressor through p53 when DNA is damaged, in result, the regulation by COX-2 on p53 will also regulate p21 [27].
Increase of COX-2 expression is able to restrain the expression of apoptosis-related gene caspase-3/7; at the same time, some experiments reveal that interference of COX-2 will enable the growth of expression of A549 cell caspase-8 and facilitate the activity of caspase-8 [28]. Caspase-9 is the essential component of the intracellular apoptosis regulation pathway, i.e. mitochondrial pathway, which is initially activated by Bcl-2 family (Bax, Bcl-2, Bcl-xL etc.). In this pathway, cytochrome C of mitochondria is released and caspase-9 is activated, thus, effector protein caspase-3 is activated, by which apoptosis is induced [29]. Increase of the caspase-9 and caspase-3 in A549 cell caused by the interference of COX-2 indicates that such interference can promote the death of lung cancer A549 cell and activate caspase-8 and caspase-9, therefore activating caspase-3. Hence, inhibition of COX-2 expression matters a lot in the prevention against occurrence and development of lung cancer [30].
COX-2 and Bcl-2 all have high expression in cancer tissue and go hand in hand; carcinogenic factors may lead cell proliferation and stimulate the expression of Bcl-2 protein, the latter of which restrain apoptosis, through up-regulating excessive expression of COX-2 and increasing synthesis of PGE2, then enabling cell proliferation and death loss balance and ultimately promoting the occurrence and development of cancer [3132]. It is found in animal experiments that highly selective COX-2 inhibitor can reduce tumor burden of cancer by increasing death of liver cancer cells; the apoptosis in the body of experimental animals is related to the significant reduction of activity of protein kinase B, expression increase of proapoptotic protein Bax and decrease of expression of inhibitor of apoptosic protein Bcl-2 [33].
There are dynamic process of the interaction between tumor cells, stromal cells and extracellular matrix, this course constitute the tumor microenvironment [34]. Fibroblasts, macrophages, and other immune cells in the tumor microenvironment secrete a variety of extracellular matrix [35]. Procollagen I, collagen I, collagen III is used as a matrix component for tumor migration or growth, and CTGF is a growth factor can stimulate fibroblast proliferation and collagen deposition [36].
TNF-α, IL-1β and iNOS are important factors for inflammation, the level of TNF-α in inflammation patients is higher than that of normal people [37]. IL-1β can stimulate diarrhea and reduce the inflammatory factors into the tissue, so as to promote the development of inflammation [38]. After inflammation develop to a certain extent can lead to cancer [39].
Celecoxib is a specific COX-2 inhibitor, it used for COX-2 inhibition in basic research and clinical treatment [40]. In this study, same concentration of celecoxib (15 µg/ml) and Res (5 µg/ml)+PA-H (10 µg/ml) had the same COX-2 inhibition activities, Res (5 µg/ml)+PA-H (10 µg/ml) might inhibit the lung cancer through their COX-2 inhibition activities.
From the result of this study, below 10 µg/ml of Res could not inhibit the growth of human fetal lung fibroblast MRC-5 cells and A549 cancer cells, and below 10 µg/mL of PA only showed growth inhibitory effects in A549 cancer cells. The combination of Res (5 µg/ml)+PA-L (5 µg/ml) and Res (5 µg/ml)+PA-H (10 µg/ml) were chosen for this study. The growth inhibitory effects of these combination were stronger than the only same concentration of PA treatment in A549 cells, and also had more apoptosic cells. These combination could decrease the mRNA and protein expressions of COX-2, VEGF, MMP-2, MMP-1, MMP-9, NF-κB, Bcl-2, Bcl-xL, procollagen I, collagen I, collagen III and CTGF, TNF-α, IL-1β, iNOS and increase the TIMP-1, TIMP-2, TIMP-3, IκB-α, p53, p21, caspase-3, caspase-8, caspase-9, Bax genes compared to the control cells and the only PA treatment. Res could enhance the anticancer effects of PA in A549 cells, thus it could be used as a anticancer intensifier for PA.
Notes
References
1. Davidson M, Smyth EC, Cunningham D. Clinical role of ramucirumab alone or in combination with paclitaxel for gastric and gastro-esophageal junction adenocarcinoma. Onco Targets Ther. 2016; 9:4539–4548. PMID: 27524910.
2. Yu B, Tan L, Zheng R, Tan H, Zheng L. Targeted delivery and controlled release of Paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Mater Sci Eng C Mater Biol Appl. 2016; 68:579–584. PMID: 27524057.

3. Cunha KS, Reguly ML, Graf U, de Andrade HH. Taxanes: the genetic toxicity of paclitaxel and docetaxel in somatic cells of Drosophila melanogaster. Mutagenesis. 2001; 16:79–84. PMID: 11139602.

4. Shi WQ. Historical story on natural medicinal chemistry of Taxol. Chinese Tradi Herbal Drug. 2011; 42:1878–1884.
5. Liu D, Kang H, Sun C, Xiao Y, Zhao K. Paclitaxel induced apoptotic genes and pathways alterations: a review. Sheng Wu Gong Cheng Xue Bao. 2013; 29:153–160. PMID: 23697160.
6. Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr. 2016; 3:8. PMID: 27148534.

7. Zulueta A, Caretti A, Signorelli P, Ghidoni R. Resveratrol: a potential challenger against gastric cancer. World J Gastroenterol. 2015; 21:10636–10643. PMID: 26457023.

8. Jang M, Pezzuto JM. Effects of resveratrol on 12-O-tetradecanoylphorbol-13-acetate-induced oxidative events and gene expression in mouse skin. Cancer Lett. 1998; 134:81–89. PMID: 10381133.

9. Bai HN, Wang ZY, Liu RH, Zhao HT, Zhang H. Synergistic ABTS radical scavenging activity of resveratrol with Auricularia auricular polysaccharides. Mod Food Sci Technol. 2014; 30:64–68.
10. Xing XM, Wang Y, Du LQ, Xu C, Lin KL, Fan SJ, Liu Q. Study on radiosensitization effect and mechanism of resveratrol on lung A549 cells. J Radiat Res Radiat Process. 2014; 32:060203.
11. Huang H, Al-Shabrawey M, Wang MH. Cyclooxygenase- and cytochrome P450-derived eicosanoids in stroke. Prostaglandins Other Lipid Mediat. 2016; 122:45–53. PMID: 26747234.

12. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016; 107:391–397. PMID: 27079437.
13. Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food. 2013; 16:9–19. PMID: 23256441.
14. Li L, Gao M, Song B, Zhang H, Wang Y. Effects of RECQ1 helicase silencing on non-small cell lung cancer cells. Biomed Pharmacother. 2016; 83:1227–1232. PMID: 27565844.

15. Feng Y, Renshaw S, Martin P. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE2. Curr Biol. 2012; 22:1253–1259. PMID: 22658594.
16. Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999; 46:407–412. PMID: 10228831.
17. Xu B, Wang Y, Yang J, Zhang Z, Zhang Y, Du H. Celecoxib induces apoptosis but up-regulates VEGF via endoplasmic reticulum stress in human colorectal cancer in vitro and in vivo. Cancer Chemother Pharmacol. 2016; 77:797–806. PMID: 26931344.

18. Li N, Zheng D, Xue J, Guo W, Shi J, Sun J, Lu C, Zheng W, Wu M, Cheng S. Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest. Exp Ther Med. 2015; 9:1709–1718. PMID: 26136881.

19. Kurihara Y, Hatori M, Ando Y, Ito D, Toyoshima T, Tanaka M, Shintani S. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clin Exp Metastasis. 2009; 26:425–432. PMID: 19241124.

20. Park SY, Kim YH, Kim Y, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012; 113:3653–3662. PMID: 22740037.

21. Abiru S, Nakao K, Ichikawa T, Migita K, Shigeno M, Sakamoto M, Ishikawa H, Hamasaki K, Nakata K, Eguchi K. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology. 2002; 35:1117–1124. PMID: 11981761.

22. Kim YI, Park SW, Yoon YK, Lee KW, Lee JH, Woo HJ, Kim Y. Orostachys japonicus inhibits the expression of MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2 genes in human PMA-differentiated THP-1 cells via inhibition of NF-κB and MAPK activation. Mol Med Rep. 2015; 12:657–662. PMID: 25760396.

23. Hilska M, Roberts PJ, Collan YU, Laine VJ, Kössi J, Hirsimäki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007; 121:714–723. PMID: 17455256.

24. Gu P, Xing X, Tänzer M, Röcken C, Weichert W, Ivanauskas A, Pross M, Peitz U, Malfertheiner P, Schmid RM, Ebert MP. Frequent loss of TIMP-3 expression in progression of esophageal and gastric adenocarcinomas. Neoplasia. 2008; 10:563–572. PMID: 18516293.

25. Wang Y, Huang G, Mo B, Wang C. Artesunate modulates expressionof matrix metalloproteinases and their inhibitors as well as collagen-IV to attenuate pulmonary fibrosis in rats. Genet Mol Res. 2016; 15:DOI: 10.4238/gmr.15027530.

26. Yodkeeree S, Pompimon W, Limtrakul P. Crebanine, an aporphine alkaloid, sensitizes TNF-α-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-κB-regulated gene products. Tumour Biol. 2014; 35:8615–8624. PMID: 24867094.

27. Gomes TS, Noguti J, Forones NM, Lima FO, Dobo C, Fernandes Junior JA, Oshima CT, Ribeiro DA. Correlation analysis of c-myc, p21(WAF/CIP1), p53, C-erbB-2 and COX-2 proteins in esophageal squamous cell carcinoma. Pathol Res Pract. 2013; 209:6–9. PMID: 23177619.

28. Kim BM, Won J, Maeng KA, Han YS, Yun YS, Hong SH. Nimesulide, a selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int J Oncol. 2009; 34:1467–1473. PMID: 19360361.

29. Singh M, Singh N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem. 2009; 325:107–119. PMID: 19191010.

30. Liu Y, Shi QF, Qi M, Tashiro S, Onodera S, Ikejima T. Interruption of hepatocyte growth factor signaling augmented oridonin-induced death in human non-small cell lung cancer A549 cells via c-met-nuclear factor-κB-cyclooxygenase-2 and c-Met-Bcl-2-caspase-3 pathways. Biol Pharm Bull. 2012; 35:1150–1158. PMID: 22791165.
31. Gallouet AS, Travert M, Bresson-Bepoldin L, Guilloton F, Pangault C, Caulet-Maugendre S, Lamy T, Tarte K, Guillaudeux T. COX-2-independent effects of celecoxib sensitize lymphoma B cells to TRAIL-mediated apoptosis. Clin Cancer Res. 2014; 20:2663–2673. PMID: 24637636.

32. Chien CC, Ko CH, Shen SC, Yang LY, Chen YC. The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells. J Cell Physiol. 2012; 227:3128–3137. PMID: 22170686.

33. Chandramohan Reddy T, Bharat Reddy D, Aparna A, Arunasree KM, Gupta G, Achari C, Reddy GV, Lakshmipathi V, Subramanyam A, Reddanna P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: downregulation of COX-2, inhibition of BCR/ABL kinase and NF-κB inactivation. Toxicol In Vitro. 2012; 26:396–405. PMID: 22245431.

34. Bracke ME, Parmar VS, Depass AL, Stevens CV, Vanhoecke BW, Mareel MM. Chick heart invasion assay. Methods Mol Biol. 2014; 1070:93–106. PMID: 24092434.

35. Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997; 76:651–660. PMID: 9166284.
36. Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008; 38:95–104. PMID: 17673689.
37. Song JL, Qian Y, Li GJ, Zhao X. Anti-inflammatory effects of kudingcha methanol extract (Ilex kudingcha C.J. Tseng) in dextran sulfate sodium-induced ulcerative colitis. Mol Med Rep. 2013; 8:1256–1262. PMID: 23969782.

38. Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998; 112:435–442. PMID: 9649212.
39. Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016; 29:54–75. PMID: 27912844.

40. Suri A, Sheng X, Schuler KM, Zhong Y, Han X, Jones HM, Gehrig PA, Zhou C, Bae-Jump VL. The effect of celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget. 2016; 7:39582–39594. PMID: 27074576.

Fig. 1
Effect of resveratrol (A), paclitaxel (B) on the growth of human fetal lung fibroblast cells MRC -5, NSCLC cell line A549 and apoptosis inducing effects (DNA content of sub-G1) of resveratrol (Res) and paclitaxel (PA) in NSCLC cell line A549 (C).
a~eMean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiplerange test. PA-L, 5 µg/ml of paclitaxel; Res+PA-L, 10 µg/ml of resveratrol+5 µg/ml of paclitaxel; PA-H, 10 µg/ml of paclitaxel; Res+PA-H, 10 µg/ml of resveratrol+10 µg/ml of paclitaxel.
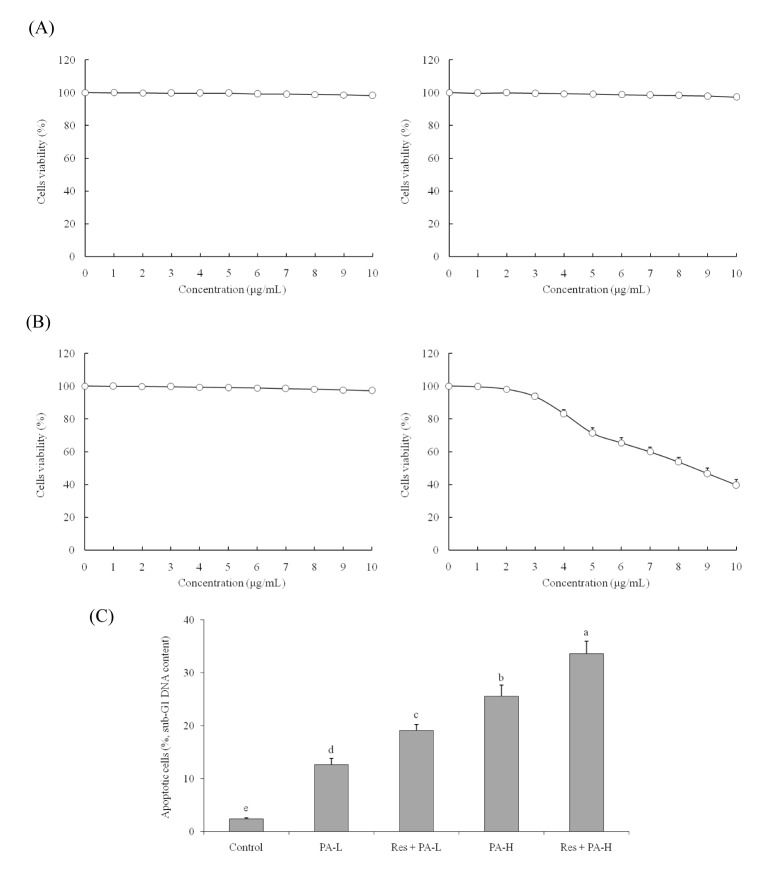
Fig. 2
The mRNA and protein expression of COX-2, VEGF (A), MMP-2, MMP-9, TIMP-1, TIMP-2 (B) and mRNA expression of MMP-1, TIMP-3 (C) in NSCLC cell line A549.
a~eMean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple-range test.
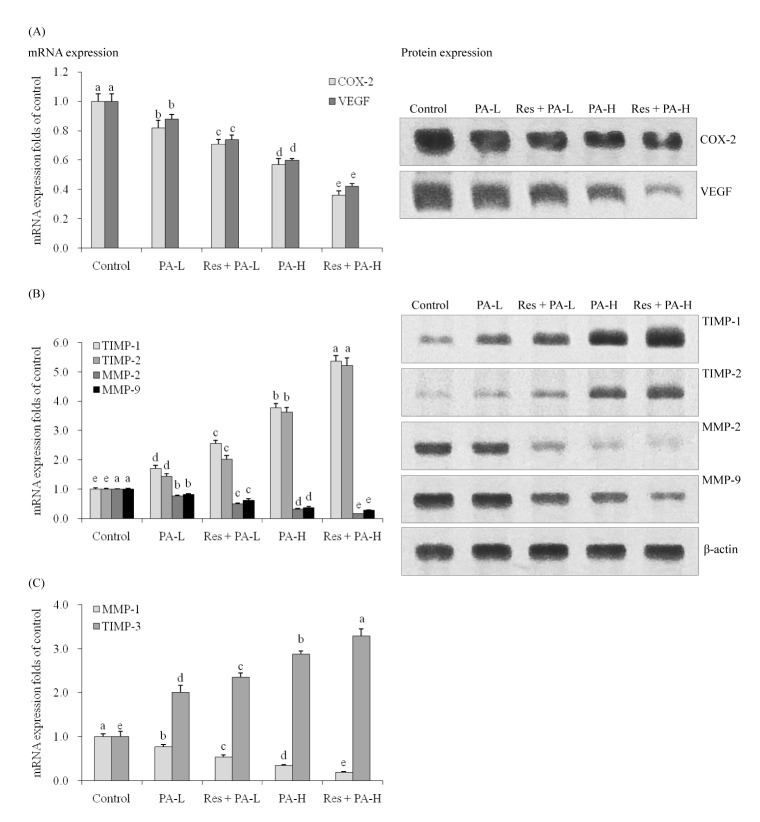
Fig. 3
The mRNA and protein expression of NF-κB, IκB-α (A), p53, p21 (B), caspase-3, caspase-8, caspase-9 (C), Bax, Bcl-2 and Bcl-xL (D) in NSCLC cell line A549.
a~eMean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple-range test.
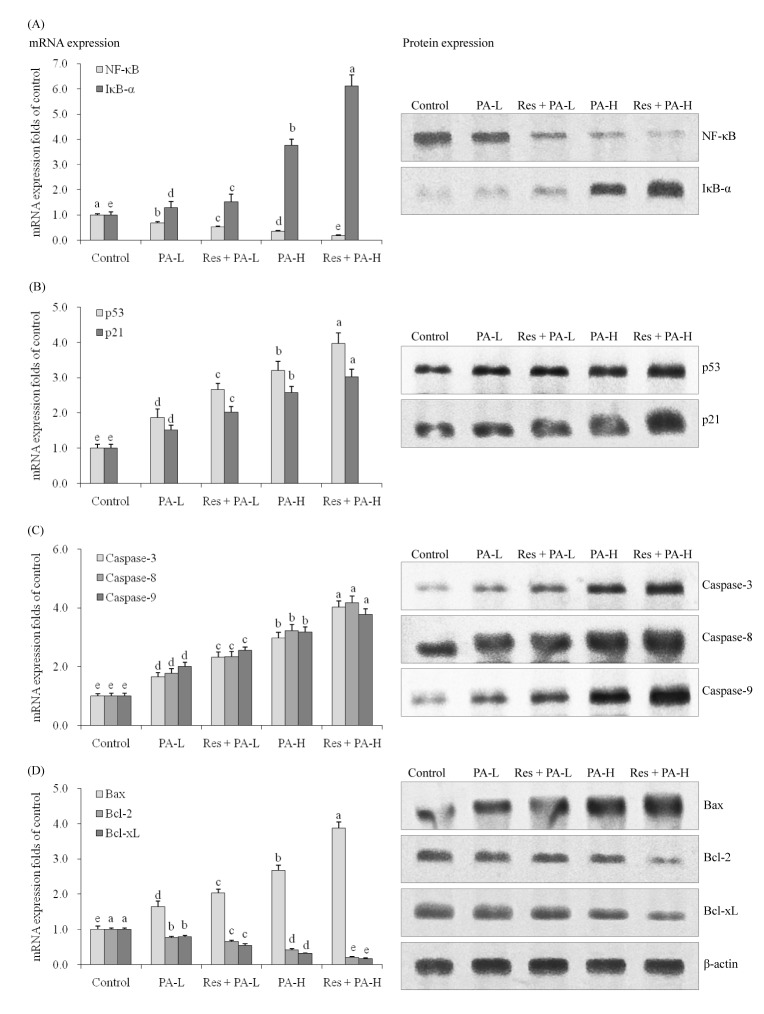
Fig. 4
The mRNA expression of procollagen I, collagen I, collagen III , CT GF (A), TNF-α, IL-1β, iNOS (B) in NSCLC cell line A549 and the mRNA expression of CO X-2 (C) in celecoxib (15 µg/ml) and Res+PA-H treated A549 cells.
a~eMean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple-range test.
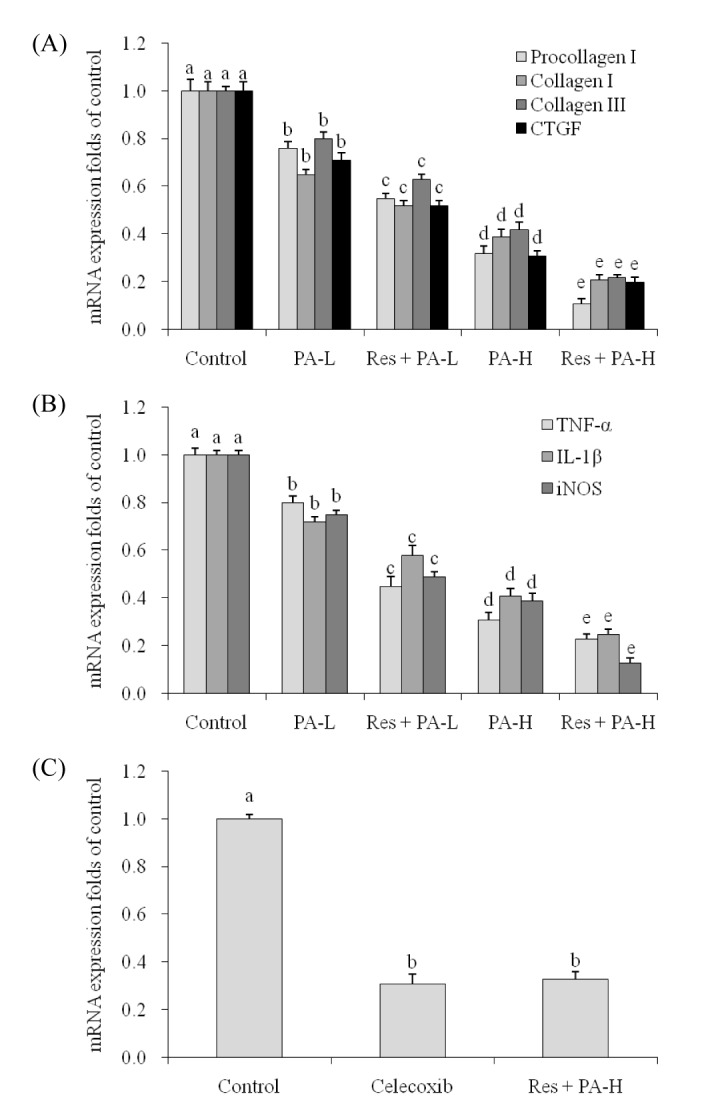
Table 2
Growth inhibition of NSCLC cell line A549 by resveratrol and paclitaxel measured by MTT assay
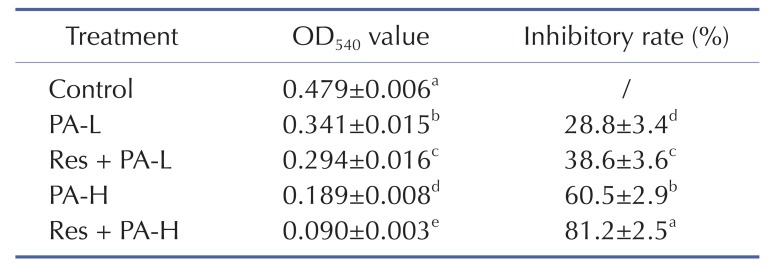
a~eMean values with different letters in the same column are significantly different (p<0.05) according to Duncan's multiple-range test.
PA-L, 5 µg/ml of paclitaxel; Res+PA-L, 10 µg/ml of resveratrol+5 µg/ml of paclitaxel; PA-H, 10 µg/ml of paclitaxel; Res+PA-H, 10 µg/ml of resveratrol+10 µg/ml of paclitaxel.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download