Abstract
This study was performed to investigate whether an intra-articular injection of transient receptor potential vanilloid 1 (TRPV1) receptor agonist, resiniferatoxin (RTX) would alleviate behavioral signs of arthritic pain in a rat model of osteoarthritis (OA). We also sought to determine the effect of RTX treatment on calcitonin gene-related peptide (CGRP) expression in the spinal cord. Knee joint inflammation was induced by intra-articular injection of monosodium iodoacetate (MIA, 8 mg/50 µl) and weight bearing percentage on right and left hindpaws during walking, paw withdrawal threshold to mechanical stimulation, and paw withdrawal latency to heat were measured to evaluate pain behavior. Intra-articular administration of RTX (0.03, 0.003 and 0.0003%) at 2 weeks after the induction of knee joint inflammation significantly improved reduction of weight bearing on the ipsilateral hindlimb and increased paw withdrawal sensitivity to mechanical and heat stimuli. The reduction of pain behavior persisted for 3~10 days according to each behavioral test. The MIA-induced increase in CGRP immunoreactivity in the spinal cord was decreased by RTX treatment in a dose-dependent manner. The present study demonstrated that a single intra-articular administration of RTX reduced pain behaviors for a relatively long time in an experimental model of OA and could normalize OA-associated changes in peptide expression in the spinal cord.
Osteoarthritis (OA) is one of the most common chronic degenerative diseases worldwide and its prevalence increases with age [12]. Disruption of articular cartilage is the pathological feature of OA that causes loss of cushioning and increased friction between exposed condyles. These events may lead to chronic arthritic pain during weight bearing and physical activity because they usually arise at weight bearing joints, such as the hip and knees [234567]. OA-induced pain has been considered a nociceptive pain that results from local tissue damage, but long-term inflammation in the synovial membrane of joints elicits subsequent modification of pain-transmitting neurons in the nervous system, which can cause a neuropathic pain condition [8].
The transient receptor potential vanilloid 1 (TRPV1), which plays important roles in the nervous system as a non-selective cation channel, a thermo-regulator, and a capsaicin receptor, has already been widely studied as a therapeutic target in several pathological pain conditions [91011]. In particular, TRPV1 and calcitonin gene-related peptide (CGRP) expression is increased by abnormally sprouting C-fiber in the synovium of the knee joint in patients with OA and in a OA rodent model [121314]. Thus, TRPV1 is a suitable target for a localized approach to minimize side effects in the management of osteoarthritic pain [12]. TRPV1 antagonists have been considered analgesics, although the fact that they cause hyperthermia may limit their effectiveness [131516].
Resiniferatoxin (RTX), an ultra-potent TRPV1 agonist, is an alternative agent that may inhibit TRPV1 activity through channel desensitization [11]. In addition, it helps to relieve inflammation temporarily because it causes local hypothermia for 7 hours following injection [17]. It has been previously reported that intra-articular administration of RTX produced analgesic effects in a rat model of rheumatoid arthritis [18].
Therefore, we investigated whether intra-articular injection of RTX produced analgesic effects in the experimental OA rat model. Changes in pain behaviors and in CGRP expression in the spinal cord were assessed to evaluate the effects of intra-articular RTX treatment.
Experiments were performed in accordance with Korea University guidelines and all animal protocols were approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC-2010-180). Male Sprague-Dawley rats (220~250 g, n=58; Orient Bio Inc., Seongnam, Korea) were used for this study. The animals were acclimated for at least 5 days prior to experiment. The rats were housed on a 12 h/12 h light/dark cycle (08:00~20:00) and were given free access to water and food.
Arthritis was induced by monosodium iodoacetate injection dissolved in saline (MIA, 8 mg/50 µl; Sigma Aldrich, St. Louis, MO, USA) in the right knee joint cavity under 4% isoflurane (Ifran LIQ, Hana Pharm., Hwasung, Korea) anesthesia using a 28 gauge syringe directly through the synovial space under the patella tendon of the right knee joint. To check that MIA injection produced arthritis, the diameters of the right and left knee joints were measured before and after injections. The knee joint diameter was defined as the distance between the lateral and medial collateral ligament regions. The first set of MIA-induced OA rats (n=18 including saline injected rats) were used to measure the time course of pain behaviors (see the next section) for 6 weeks and to determine suitable timing for RTX administration.
Behavioral tests were performed 1 day before (pre) and 4 hours, 1, 3, 5, 7, 10, 14 and 21 days after RTX treatment in OA rats. The investigator conducting the behavioral tests was blinded to the identity of the injected drug.
Weigh bearing test: Measurement of weight bearing was done using a weight bearing device that allowed for assessment of arthritic pain in freely walking rats [19]. This allowed for the measurement of weight load on each limb while the animal was walking through a path, the bottom of which was equipped with strain gauge weight sensors (DACELL, Cheongju, Korea). The output of each load cell was fed to a digital amplifier (DN-AM 310, DACELL, Cheongju, Korea) for appropriate amplification and filtering. The processed signal was sent to a personal computer via an analog-digital converter (PCM1716, Texas instrument, Texas, USA) and plotted as a time-weight curve using a software program we designed (WBT, Korea University, Seoul, Korea). The test was repeated until at least five time-weight curves were obtained for a given limb. Weight load borne by the ipsilateral limb was expressed as a percentage of weight borne by both hind limbs.
Mechanical sensitivity: The rats were placed in transparent plastic cages (28×28×10 cm) on a metal mesh floor, and a series of 8 different von Frey filaments (0.41~15.10 g, Stoeling, Wood Dale, IL USA) was applied for 2~3 seconds to the plantar surface of the ipsilateral hind paw. The threshold for eliciting withdrawal 50% of the time was determined using an up-down method [20] while initiating the experiment with a filament that had a strength of 2.0 g (4.31 mN). Stimuli were presented at intervals of several seconds. Interpolation of the 50% threshold was carried out according to the method of Dixon [21].
Thermal sensitivity: The withdrawal latency to heat stimulation was measured with the Hargreaves apparatus (Ugo Basile, Varese, Italy), which was recorded automatically when an animal paw escaped from an I.R. source (heat stimulator) by a sensor. A cutoff time of 15 seconds was applied to protect potential tissue damage. Each test was repeated three times and had 15 min interval between tests. Latencies from the three times were averaged [22].
Timing of RTX injection (2 weeks after MIA injection) was determined based on the time course of pain behaviors after MIA injection. At that time, pain behaviors were fully developed and maintained for next 3 weeks. After MIA injection, each rat was randomly assigned to one of four groups (n=6 per group: 0.0003%, 0.003% and 0.03% RTX groups and vehicle group). The 1 mg of RTX (Sigma Aldrich, ST. Louis, MO, USA) was dissolved in 1 ml of 95% ethanol (stock solution) and then diluted in 0.9% saline with 3% tween 80 for each concentration. In a vehicle group, 30% ethanol with 3% tween 80 was injected. Doses of RTX were determined according to a previous report [18]. RTX was injected at a volume of 50 µl using a 28 gauge syringe into the right knee joint cavity under 4% isoflurane anesthesia. Bupivacaine (0.5%, 10 µl) was intra-articularly administrated 10 min before RTX injection to preclude the initial excitatory effect of RTX that has been previously reported [18].
Animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, IP) and perfused with 4% paraformaldehyde (pH 7.4). The segments of the spinal cord at lumbar 3~5 were collected 5 days after RTX treatment in MIA-induced OA rats (n=4/group). After cryopreservation with 30% sucrose, tissues were embedded in optical cutting temperature (OCT) compound and then were sectioned at 14-µm thickness in a cryostat (HM550, Thermo Scientific, Walldorf, Germany). Sections were incubated with rabbit anti-CGRP (1:8000, PC205L, Merck Millipore, Darmstadt, Germany) for 48 hours at 4℃ and then incubated with biotinylated anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA, USA) for an hour at 4℃. Sections were stained with 0.02% 3,3'-diaminobenzidine (DAB, SK-4100, Vector Laboratories, Burlingame, CA, USA) adding 0.01% H2O2 for 4 min. Control experiments were performed without primary or secondary antibodies to identify the background signal levels. The stained sections were examined with a Leica DM 2500 (Leica Microsystems GmbH, Wetzlar, Germany) microscope, and images were captured with a Leica Camera DFC 450C (Leica Microsystems GmbH). The lamina I-III region of the spinal cord was selected and then measured densities of CGRP immunoreactivity using Scion image software.
All values were expressed as the mean±SEM. All statistical tests were evaluated at an alpha level of 0.05. We used the Friedman repeated measures of analysis of variance (ANOVA) test followed by multiple comparison tests to analyze behavioral test results before and after MIA injection and RTX administration in the same animal. Comparison of the relative intensities of CGRP immunoreactivity between RTX treated groups and vehicle group was performed using a one-way ANOVA with Tukey's HSD post hoc test.
Knee joint diameters significantly increased after MIA injections (Fig. 1A). The diameter was 10.6±0.5 mm before MIA injection. The increase in diameter reached its peak on the first day (15.7±1.2 mm) and gradually decreased. The diameter was 12.3±0.5 mm at 7 days after MIA injection and was maintained until the end of observation. Knee joint diameters were significantly increased compared to values obtained from control values up to day 42. Weight bearing was measured as the potential index of non-evoked pain [2324]. Following MIA injection, rats tried to reduce the weight support on the ipsilateral hindlimb probably due to the pain and discomfort in the knee joint. Rats showed that they could not put weight on their ipsilateral hindlimb and had to hop to avoid pressure applied into the joint during the test. A weight bearing capacity of the ipsilateral hindlimb abruptly decreased in during locomotion and a slow recovery toward normal (Fig. 1B). Weight bearing on the ipsilateral hindlimb was around 55% before MIA injection and was reduced to 37.7±1.7 % on the first day and worsened up to the fifth day after which it slowly recovered. Weight bearing was significantly different from control values up to day 35. Mechanical allodynia was well developed after MIA injection (Fig. 1C). The paw withdrawal threshold (PWT) decreased to 6.6 g±2.5 g at day 1 and gradually decreased up to the end of the experiment, where the withdrawal threshold was 3.3 g±0.9 g. The paw withdrawal threshold was significantly reduced compared to pre-inflammatory control values. Paw withdrawal latency (PWL) to heat stimuli decreased after MIA injection to 6.8±0.8 seconds at day 1, which was not significantly different from pre-injection value, but differed from control values from day 7 onward (Fig. 1D).
To test the analgesic effects of locally administered RTX into the knee joint cavity on chronic arthritic pain-like behaviors, such as reduction of weight bearing and paw withdrawal sensitivity to mechanical and thermal stimuli in MIA injected rats, RTX (0.0003%, 0.003% and 0.03% RTX) was injected 14 days after MIA injection. RTX injection timing was determined based on the results of MIA injection groups.
Weight bearing: Intra-articular RTX injection significantly reversed MIA-induced reduction of weight load in the ipsilateral hindlimb only in the highest dose (0.03%) group for 3 days (Fig. 2A). RTX at a concentration of 0.003% appeared to improve the reduction of weight bearing, but these changes were not statistically significant. RTX at the smallest dose did not result in any changes in weight bearing (Fig. 2A).
Paw withdrawal sensitivity to mechanical stimuli: Intra-articular RTX (0.03%, 0.003% and 0.0003%) produced a dose dependent elevation of the decreased PWT in the MIA-injected rats (Fig. 2B). In detail, RTX at a concentration of 0.03% significantly increased PWT from 4 hours to 10 days compared to the value obtained pre-RTX injection, but PWT temporally reverted to pre-injection level at 1 day after injection. RTX at concentrations of 0.003% and 0.0003% significantly increased the PWT for 3 days compared to the pre-injection value. Rats that received intra-articular injection of bupivacaine alone were tested for 4 hours. The PWT was significantly elevated compared to the pre-injection value for 2.5 hours and then returned to pre-injection level. The first test point after RTX treatment in this study, 4 hours after the injection, was determined based on this result.
Paw withdrawal sensitivity to heat stimuli: All three doses of intra-articular RTX (0.0003%, 0.003% and 0.03%) showed a dose-dependent elevation of the decreased PWL by MIA. The PWL was significantly increased from 4 hours to 10 days in the 0.003% and 0.03% RTX-treated groups and for 3 days in the lowest dose (0.0003%) group (Fig. 2C).
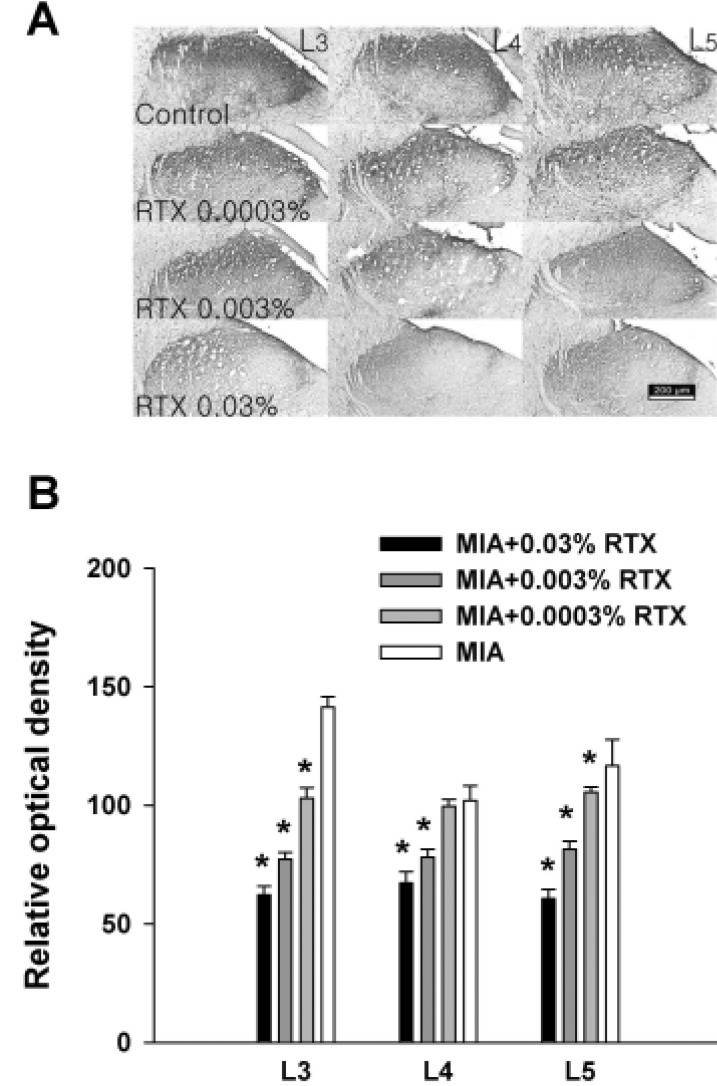
CGRP was stained in the spinal segments of L3, L4 and L5 5 days after RTX administration. CGRP immunoreactivity was detected in the dorsal horn of the spinal cord (Fig. 3A). All dosages of RTX decreased the CGRP immunoreactivity in a dose-dependent manner in the spinal segments except in the L4 spinal cord of the 0.0003% RTX treated group we observed (Fig. 3B).
The major finding of this study is that a single, intra-articular administration of RTX produces long-lasting analgesic effects in an experimental OA rat model. Localized injection of RTX into the knee joint cavity alleviated pain behaviors for 3~10 days according to each pain behavior and decreased CGRP immunoreactivity in the dorsal horn of the spinal cord which was increased in the inflammatory condition.
In the present study, we used 8 mg of MIA which produced severe osteoarthritis in rats' knees. Intra-articular injected monosodium iodoacetate (MIA) disrupts chondrocyte metabolism and produces cartilage degeneration through irreversible inhibition of a glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [25]. The MIA-induced severe osteoarthritis model is complicated by chronic inflammation and destruction of articulate [26]. Inflammatory exudation and synovial infiltration appear on the first day after intra-articular MIA injection [27]. At this time point in our study, the ipsilateral knee joint was excessively swollen. Swelling gradually subsided, but a difference compared to saline-injected rats was maintained. Continuous inflammation in the knee joint activates nociceptors and that leads to hyper-excitation of secondary neurons in the spinal cord, called central hypersensitivity [2829]. Thus, drugs targeting the spinal cord can attenuate pain behaviors in the MIA model [30]. According to previous reports [313233], sensory information in the knee joint is transmitted to the L3-5 spinal cord segments. Consequently, secondary hyperalgesia, such as increased mechanical sensitivity in the plantar surface of the hindlimb observed in this study, is manifested as a result of central sensitization. In addition, MIA destroyed subchondral bone and synovium resulting from MIA, as seen in OA in humans, is the most important factor in generating OA pain [2534]. Thus, we assessed the weight load bearing during freely walking which reflects human OA pain in the ipsilateral hind limb [19]. Following MIA injection, weight bearing was significantly decreased in the ipsilateral hind-limb from 1 day after MIA. Paw hypersensitivity to mechanical and thermal stimuli was also increased at the same time.
Primary afferent nociceptors are divided into peptidergic and non-peptidergic neurons. In contrast to the non-peptidergic neurons, most of the peptidergic nociceptors express TRPV1 [3536]. In osteoarthritic patients and rodents, abnormal sprouting of C-fiber, which is produced by neurogenic inflammation, is found at the synovium of the knee joint [1214]. Density of CGRPimmunoreactive fibers is also increased at the ipsilateral side of the synovial compartments in the inflamed joint [1437]. Thus, we tried to block activation of nociceptors in the knee joint by peripheral TRPV1 inhibition. A single injection of capsaicin causes initial neuronal excitation, but repeated application produces desensitization of TRPV1 resulting in analgesic effects [38]. On the other hand, RTX, which is almost 1000 times more potent than capsaicin, is strong enough to trigger agonist-induced desensitization, and is verified as a TRPV1 blocker in adults [1139]. In addition, RTX is widely reported to have analgesic effects in several pathological pain conditions, including rheumatoid arthritis and neuropathic pain [40]. In this study, administration of a single dose of RTX into the knee joint alleviated non-evoked pain (weight bearing) as well as evoked pain (mechanical and thermal sensitivities), showing that a single peripheral RTX injection has long-lasting analgesic effects. The analgesic efficacy or duration of RTX on each pain behavior differs between pain behaviors. Only the highest dose of RTX increased mechanical and thermal sensitivities for 10 days, but it ameliorated weight bearing measurement for 3 days. At present, the source of this discrepancy is unknown, but the nature of each pain behavior may be at least in part responsible.
In this study, RTX increased mechanical PWT at 4 hours, and reverted on the next day (see Fig. 2A). Although we cannot explain exactly why that was reverted, we speculate that local destruction of TRPV1 expressing fibers by RTX injection evokes inflammation. RTX destroys TRPV1 expressing fibers, which is almost C-fibers and 30% of A-fibers. Unlike withdrawal response to heat stimulation, withdrawal response to mechanical stimulation depends on unmyelinated fibers and myelinated fibers. However, further study is needed to clarify detailed mechanism.
Weight bearing measurement is considered to reflect non-evoked and walking-induced pain or discomfort [23]. Moreover, weight bearing on the ipsilateral limb is around 55% in normal rats and is decreased to 45% 14 days after MIA injection. Therefore, the margin of potential improvement in this pain behavior measurement is small. In contrast, paw withdrawal threshold to mechanical stimulation and paw withdrawal latency to heat stimuli represent behavioral signs of secondary hyperalgesia and evoked response to obvious external stimuli. Paw withdrawal threshold and latency in normal rats were around 15 g and 11 seconds, respectively and reduced to around 3 g and 6 seconds 14 days after MIA injection, respectively. Therefore, the margin of potential improvement in these pain behaviors is large.
Previous studies reported that spinal CGRP is increased in MIA induced OA rats [4142]. In this study, we observed that increased CGRP expression in the dorsal horn of the lumbar spinal cord was decreased after intra-articular RTX injection. Capsaicin induced TRPV1 activation increases more calcium uptake and it causes CGRP release [43]. However, RTX is 1000 times stronger than capsaicin enough to destroy unmyelinated c-fiber containing TRPV1. Loss of these c-fiber interrupts the transmission of pain and inflammatory information [44]. Consequently, CGRP production in DRG reduces, and that leads to decreased CGRP-IR in the spinal cord. We predict that reduced peripheral nociceptive inputs by RTX leads to change the spinal excitation through reduction of CGRP release. In general, a deleted C-fiber would be re-innervated at the knee joint after an analgesic period. In this study, decreased pain behaviors were rekindled around 10 days after RTX treatment. This analgesic period is slightly shorter than examples in previous reports utilizing systemic or direct sensory ganglion injections [1117].
Locally-administered RTX is more useful than systemic injection because it prevents the unnecessary deletion of C-fiber in the non-target tissues. OA prevalence is increased in elderly individuals, who are also more likely to take medication for other diseases. Therefore, local delivery of this drug may be beneficial to OA patients. However, the major problem with RTX treatment is acute toxicity. In our study, we blocked this using the local anesthetics, bupivacaine. It is critical to alleviate acute burning pain that can accompany RTX treatment, even if this drug produces long-lasting analgesic effects down the line. If this issue is resolved, local RTX treatment may be an effective solution to lighten the burden of osteoarthritis pain.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) 2010-0006489 and 2011-0005415. It was also partially supported by a grant of the Korea Institute of Industrial Technology (KITECH).
Notes
Author contributions: Y.K., Y.W.Y and J.K. contributed to the conception and design of this study. E.K., K.S.L., K.L., and S.H.N. performed the behavioral tests experiments. E.K. and S.H.P. performed the assay for CGRP expression. Y.K. C.K. and J.K. wrote the manuscript. J.K. and Y.W.Y. supervised and coordinated the study.
References
1. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014; 73:1323–1330. PMID: 24553908.

2. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010; 26:355–369. PMID: 20699159.

3. Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006; 14:13–29. PMID: 16242352.

4. Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010; 1192:230–237. PMID: 20392241.

5. Kim YM, Joo YB. Patellofemoral osteoarthritis. Knee Surg Relat Res. 2012; 24:193–200. PMID: 23269956.

6. Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013; 15:223. PMID: 24321104.

7. Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011; 2011:683970. PMID: 22046522.

8. Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014; 10:374–380. PMID: 24686507.

9. Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Ther Adv Neurol Disord. 2014; 7:22–32. PMID: 24409200.

10. Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005; 25:5109–5116. PMID: 15917451.

11. Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004; 113:1344–1352. PMID: 15124026.

12. Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005; 388:75–80. PMID: 16039054.

13. Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, McWilliams DF, Okine BN, Barrett DA, Hathway GJ, Walsh DA, Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015; 74:252–259. PMID: 24152419.

14. Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to painrelated behavior in arthritis. J Neurosci. 2013; 33:10066–10074. PMID: 23761902.

15. Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006; 26:9385–9393. PMID: 16971522.

16. Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008; 136:202–210. PMID: 18337008.

17. Abdelhamid RE, Kovács KJ, Honda CN, Nunez MG, Larson AA. Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors. J Pain. 2013; 14:1629–1641. PMID: 24188863.

18. Kissin EY, Freitas CF, Kissin I. The effects of intraarticular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005; 101:1433–1439. PMID: 16244007.

19. Min SS, Han JS, Kim YI, Na HS, Yoon YW, Hong SK, Han HC. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett. 2001; 308:95–98. PMID: 11457568.

20. Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997; 280:829–838. PMID: 9023297.
21. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.

22. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88. PMID: 3340425.

23. Lee KS, Kim J, Yoon YW, Lee MG, Hong SK, Han HC. The peripheral role of group I metabotropic glutamate receptors on nociceptive behaviors in rats with knee joint inflammation. Neurosci Lett. 2007; 416:123–127. PMID: 17314010.

24. Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain. 2007; 3:8. PMID: 17391515.

25. Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Monoiodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003; 31:619–624. PMID: 14585729.

26. Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y, Kubo T, Takahashi K, Ohtori S. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011; 12:134. PMID: 21679434.
27. van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol. 1989; 135:1001–1014. PMID: 2556924.
28. Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011; 7:94. PMID: 22145886.

29. Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat's knee. J Neurophysiol. 1990; 64:299–311. PMID: 2388073.

30. Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011; 493:72–75. PMID: 21241772.

31. Ferreira-Gomes J, Adães S, Sousa RM, Mendonça M, Castro-Lopes JM. Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat. Mol Pain. 2012; 8:50. PMID: 22769424.

32. Hirasawa Y, Okajima S, Ohta M, Tokioka T. Nerve distribution to the human knee joint: anatomical and immunohistochemical study. Int Orthop. 2000; 24:1–4. PMID: 10774852.

33. Widenfalk B, Wiberg M. Origin of sympathetic and sensory innervation of the knee joint. A retrograde axonal tracing study in the rat. Anat Embryol (Berl). 1989; 180:317–323. PMID: 2478046.
34. Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, Kazis L, Gale DR. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001; 134:541–549. PMID: 11281736.

35. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.

36. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998; 21:531–543. PMID: 9768840.

37. Murakami K, Nakagawa H, Nishimura K, Matsuo S. Changes in peptidergic fiber density in the synovium of mice with collagenaseinduced acute arthritis. Can J Physiol Pharmacol. 2015; 93:435–441. PMID: 25909759.

38. Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008; 24:142–154. PMID: 18209521.
39. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999; 51:159–212. PMID: 10353985.
40. Wendler J, Burmester GR, Sörensen H, Krause A, Richter C, Tony HP, Rubbert-Roth A, Bartz-Bazzanella P, Wassenberg S, Haug-Rost I, Dörner T. Rituximab in patients with rheumatoid arthritis in routine practice (GERINIS): six-year results from a prospective, multicentre, non-interventional study in 2,484 patients. Arthritis Res Ther. 2014; 16:R80. PMID: 24670196.

41. Ferland CE, Pailleux F, Vachon P, Beaudry F. Determination of specific neuropeptides modulation time course in a rat model of osteoarthritis pain by liquid chromatography ion trap mass spectrometry. Neuropeptides. 2011; 45:423–429. PMID: 21855139.

42. Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, Lewis LG, Bianchi BR, Mikusa JP, Koenig JR, Perner RJ, Kort ME, Honore P, Faltynek CR, Kym PR, Reilly RM. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydronaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010; 150:319–326. PMID: 20621685.

43. Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009; 29:4981–4992. PMID: 19369567.

44. Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015; 156:1018–1024. PMID: 25659068.
Fig. 1
Changes in knee joint diameter (A), weight bearing distribution (B) and paw withdrawal responses to mechanical (C) or thermal stimuli (D) in the ipsilateral side of the hind paw after 8 mg of intra-articular MIA injection.
Asterisks indicate the values that were significantly different from the value obtained in the pre-MIA injection period (P, p<0.05). Data are expressed as mean±SEM.
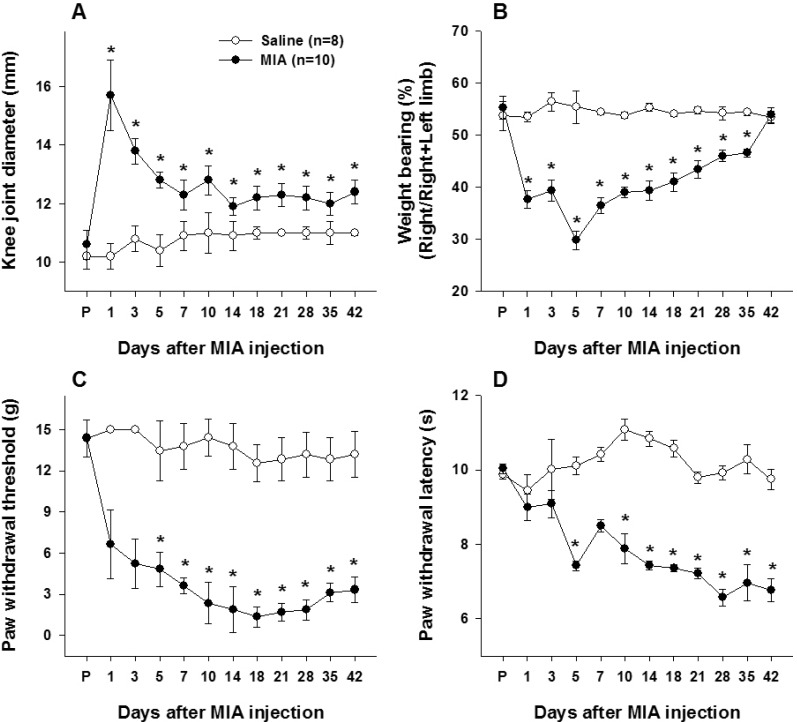
Fig. 2
Effect of intra-articular resiniferatoxin on weight bearing measurement (A), paw withdrawal responses to mechanical (B) and thermal stimuli (C) in the ipsilateral side of the hind paw following MIA injection.
RTX was administrated 14 days after MIA injection. Asterisks indicate the values that were significantly different from the value obtained pre-RTX injection period (P). Data are expressed as mean±SEM.
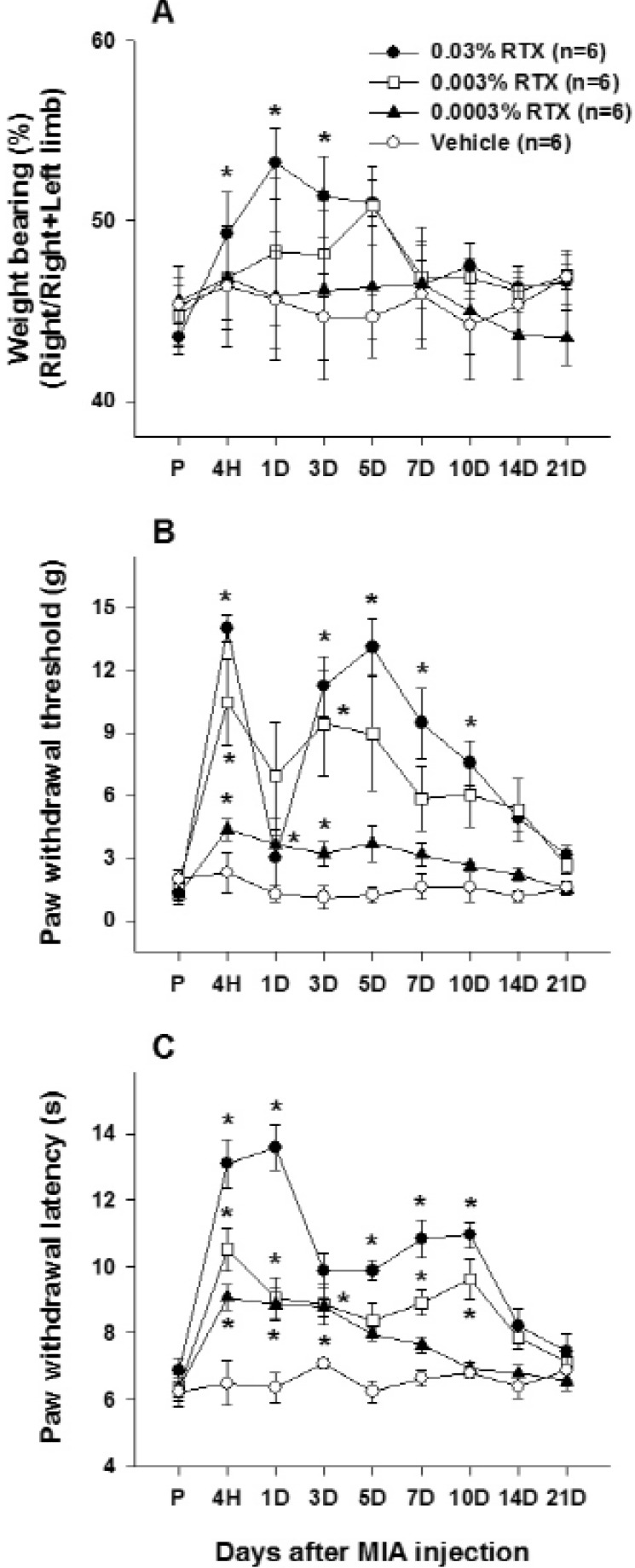
Fig. 3
(A) A representative section showing CGRP immunoreactivity in the dorsal horn of L3, L4 and L5 spinal cord.
Transverse sections of the L3-L5 spinal cord segments were stained with anti-CGRP antibody in each group, scale bar: 200 µm. (B) Relative optical density of CGRP immunoreactivity in each group. Asterisks indicate the values that were significantly different between the RTX treated groups and MIA control group (p<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download