Abstract
Recently, Cynanchi wilfordii Radix has gained wide use in Asian countries as a functional food effective for relieving fatigue, osteoporosis, and constipation, particularly in menopausal disorders. However, its anti-inflammatory and anti-microbial activities have not been explored in detail to date. The anti-inflammatory, antioxidant, and anti-bacterial properties of the Cynanchi wilfordii Radix extracts obtained with water, methanol, ethanol, and acetone were compared. All 4 polyphenol-containing extracts exhibited anti-inflammatory and antioxidant effects. The ethanol extract was found to elicit the most potent reduction of nitric oxide (NO), prostaglandin E2 (PGE2), and cytokine (IL-1β, IL-6, IL-10, and TNF-α) levels, as well as inhibit the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in a concentration-dependent manner. The evaluation of antioxidant activity also revealed the ethanol extract to have the highest free radical scavenging activity, measured as 85.3±0.4%, which is equivalent to 99.9% of the activity of α -tocopherol. In the assessment of anti-bacterial activity, only ethanol extract was found to inhibit the growth of the Bacillus species Bacillus cereus and Bacillus anthracis. These results show that polyphenols of Cynanchi wilfordii Radix have anti-inflammatory, antioxidant, and anti-bacterial properties that can be exploited and further improved for use as a supplementary functional food, in cosmetics, and for pharmaceutical purposes.
There is considerable research interest in the identification of new anti-inflammatory, antioxidant, and anti-microbial agents from plants used in traditional medicine. While 80% of the world's population relies on traditional medicines for their primary care needs [1], very few plants are currently used as anti-inflammatory, antioxidant, and anti-microbial agents.
Cynanchi wilfordii Radix has been used in Korean Oriental medicine for the prevention and treatment of various geriatric diseases involving vascular disorders, including diabetes mellitus, ischemia-induced diseases, and the progression of aging [2]. It is widely used as an effective functional food for management of fatigue, osteoporosis, and constipation, particularly in menopausal disorders. Cynanchi wilfordii Radix, a plant belonging to the Asclepiadaceae family, is commonly used in Oriental medicine. It is a rich source of pregnane glycosides [3] and biologically active compounds known to modulate cardiac function [4]. To date, this plant has been used to improve renal, cardiac [5], and hepatic function; reduce fatigue [6]; and strengthen the bones and muscles. However, the anti-inflammatory and anti-bacterial effects of Cynanchi wilfordii Radix have not been reported to date.
Within the inflammatory response, macrophages are critical immune cells that regulate the inflammatory cascades. The immune response to microbial pathogens relies on both innate and adaptive immune responses. Activated macrophages secrete a number of inflammatory mediators, including interleukin (IL)-1β, IL-6, IL-10, prostaglandin E2 (PGE2), nitric oxide (NO), and tumor necrosis factor-α (TNF-α) [7,8,9]. Cytokines such as IL-1β, IL-6, IL-10, and TNF-α are soluble proteins that are secreted by the cells of the immune system [10]. Free radicals, in the form of reactive oxygen and nitrogen species, are an integral part of normal physiological processes. Overproduction of these reactive species can occur due to oxidative stress brought about by an imbalance between the antioxidant defense system of the body and free radical formation [11,12].
Food poisoning is accompanied by diarrhea and emesis, and is associated with gastroenteritis [13,14]. Human infection can result from the consumption of contaminated dairy products, poultry, dried or frozen eggs, and shellfish from contaminated water [15]. The Escherichia coli O157: H7 bacteria is widely recognized to be involved in food poisoning and is considered to be the main cause of life-threatening complications, including hemorrhagic colitis and hemolytic uremic syndrome in children and immunocompromised patients [16,17]. Bacillus cereus, one of the most important bacteria involved in instances of poisoning, is recognized as a causative agent of food poisoning and gastroenteritis [18,19]. Bacillus anthracis, a notorious biological warfare agent, has been used as a weapon with the intent to cause substantial morbidity and mortality, potentially crippling an entire city or region [20]. In this study, extracts from Cynanchi wilfordii Radix were prepared using 4 different solvents (water, methanol, ethanol, and acetone) and their anti-inflammatory and anti-bacterial potential were determined.
Solvents (methanol, ethanol, and acetone) were obtained from Samchun Pure Chemical Co., Ltd. (Pyeongtaek, Korea). Cynanchi wilfordii Radix was purchased from an Oriental drug store in Seoul, Korea. Folin-Ciocalteu reagent, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reagent, Griess reagent, lipopolysaccharide (LPS), gallic acid, α-tocopherol, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine albumin (FBS), and gentamycin were purchased from GIBCO BRL (Grand Island, NY, USA). Enzyme-linked immunosorbent assay (ELISA) kit and Mueller-Hinton agar were purchased from R&D Systems (Minneapolis, MN, USA) and Difco (Detroit, MI, USA), respectively.
Dried Cynanchi wilfordii Radix (20 g) was extracted with hot water and 80% methanol, 80% ethanol, or 80% acetone under reflux for 24 h, and filtered using Whatman filter paper (Macherey-Nagel; Düren, Germany). The filtered supernatants were evaporated using a rotary evaporator (Eyela; Tokyo, Japan) under reduced pressure, yielding a viscous solution. The concentrated extracts were freeze-dried and stored in the dark at 4℃ until the time of the experiments.
Total polyphenol content (TPC) of the extracts was measured using the Folin-Denis method [21]. Folin-Ciocalteu reagent (750 µl) was added to 150 µl of the sample and the reaction mixture was incubated for 5 min. After the incubation, 600 µl of 10% Na2CO3 was added and the reaction mixture was incubated at room temperature for 60 min. Absorbance of the reaction mixture was subsequently measured at 760 nm using Infinite® F200 plate reader (Tecan, Männedorf, Switzerland). Gallic acid was used to prepare the standard curve.
Murine RAW 264.7 cells were obtained from the Korea Cell Bank (Seoul, Korea) and cultured in DMEM containing 10% FBS, 20 µg/µL of gentamycin at 37℃ in a CO2 incubator (Model 3111, Thermo Fisher Scientific; Waltham, MA, USA). The cells were seeded at 5×105 cells/well in 24-well plates. Cell viability was tested following incubation with 0, 1, 5, 10, 50, 100, and 250 µg/µl concentrations of each extract. After 24 h of incubation, the cells were subjected to an MTT assay. Medium was replaced with 500 µl of serum-free media with MTT (0.5 mg/µl). After incubation for 1 h, the medium was removed and solubilized by adding 500 µl of DMSO to each well. The absorbance was monitored at a 590 nm wavelength using a microplate reader.
RAW 264.7 cells were plated in 24-well cell culture plates with 1 ml of culture medium and incubated for 24 h. The cells were then treated with 0, 1, 5, 10, 50, 100, and 250 µg/µl of each extract with 0.1 µg/µl of LPS, and incubated for 24 h. The nitrite generated was quantitated using the Griess reagent system. The supernatant was mixed with an equal volume of Griess reagent and incubated for 10 min at room temperature. The absorbance was measured at 540 nm using a microplate reader and nitrite concentration was determined.
The PGE2, IL-1β, IL-6, IL-10, and TNF-α levels were determined in the supernatants from RAW 264.7 cells cultures using an ELISA kit according to the manufacturer's instructions. The cells were incubated with LPS (0.1 µg/µl) in the presence of a range of concentrations of Cynanchi wilfordii Radix extracts for 24 h. The absorbance was measured at 450 nm using a microplate reader and a standard curve was used to determine the levels of each cytokine.
RAW 264.7 cells were incubated with LPS (0.1 µg/µl) in the presence of a range of concentrations of Cynanchi wilfordii Radix extracts for 24 h. Total protein was extracted from cultured cells using the freeze-thaw lysis method. Equal amounts of protein from each sample were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes (Bio-Rad; Hercules, CA, USA). After blotting, the membrane was incubated with specific primary antibody overnight at 4℃, and subsequently incubated for 1 h with a secondary antibody. Protein signals on the membrane were quantified using a luminescent image analyzer LAS-4000 mini (Fujifilm Life Sciences; Tokyo, Japan).
Antioxidant activity was evaluated using 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) free radical scavenging activity according to the method described by Hatano and Kagawa [22]. α-Tocopherol reagent (Sigma-Aldrich; St. Louis, MO, USA) was used as a positive control. Each extract was mixed with 100 µl of DPPH solution to a final concentration of 0.2 mM and allowed to stand for 30 min. The absorbance of the mixture was measured at 517 nm using a microplate reader. Scavenging activity was calculated using the following equation. where Atest represents the absorbance of the test solution, and Acontrol represents the absorbance of the α-tocopherol solution.
Anti-bacterial activity against 5 pathogens commonly involved in food poisoning was determined by the agar disc diffusion method using the measurement of the diameter of the clear zone. Bacillus cereus KCTC 3624T was obtained from the Korean Collection for Type Cultures (KCTC; Daejeon, Korea). Bacillus anthracis ATCC 14578T and Escherichia coli O157:H7 ATCC 43894 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Salmonella typhimurium NCCP 14774 was obtained from the National Culture Collection for Pathogens (NCCP; Cheongwon, Korea). Vibrio cholerae O139 was obtained from the Department of Microbiology, College of Medicine, Chonnam National University (Gwangju, Korea). The strains were grown aerobically on Mueller-Hinton agar at 37℃ for 18 h. Cultured media from test microorganisms were placed in sterilized paper discs (10 mm diameter) with each extract and placed on the inoculated agar surface. A broth micro-dilution susceptibility assay was conducted in accordance with NCCLS 2009 [23] guidelines for the minimal inhibitory concentration (MIC) determinations. MIC values were defined as the lowest concentrations of Cynanchi wilfordii Radix extracts where no visible growth can be observed.
All measurements were performed in triplicate and the results are expressed as means±standard error (SE). Statistical analyses of the differences between samples were carried out using one-way analysis of variance (ANOVA) followed by a post-hoc correction for multiple comparisons using the Duncan test and the Student's t-test using the Predictive Analytics Software (PASW) statistics package for Windows. p<0.01 was considered statistically significant.
The amount of TPC determined with 1,000 µg/ml of each of Cynanchi wilfordii Radix extract is shown in Table 1. TPC concentrations in water, methanol, ethanol, and acetone extracts were determined to be 5.6±0.6, 10.0±0.3, 19.8±6.4, and 10.7±0.6 µg gallic acid equivalents (GAE)/g, respectively. The highest TPC was detected in the ethanol extract.
The cytotoxic effects of polyphenols in Cynanchi wilfordii Radix extracts were evaluated in RAW 264.7 cells using the MTT assay. Polyphenols were measured at treatment concentrations in the cell culture system. Cells were treated for 24 h with 0, 1, 5, 10, 50, 100, or 250 µg/mL of each Cynanchi wilfordii Radix extract. Control group cells were treated with media only. As shown in Fig. 1, no cytotoxic effects of polyphenols (up to a concentration of 250 µg/ml) were noted after a 24-h incubation.
The inhibitory effects of 4 extracts of Cynanchi wilfordii Radix on the production of nitrite, a stable metabolite of NO, and PGE2 were evaluated and compared in LPS-challenged RAW 264.7 cells. Cells were treated for 24 h with 0, 1, 5, 10, 50, 100, or 250 µg/ml of each extract in the presence of 0.1 µg/ml LPS. As shown in Fig. 2, Cynanchi wilfordii Radix extracts effectively suppressed LPS-induced nitrite production in a dose-dependent manner. Consistent with the observed nitrite accumulation, the extracts profoundly inhibited PGE2 production. NO production in cells treated with the ethanol extract was significantly lower than that in cells treated with the water, methanol, or acetone extracts at 50 and 100 µg/ml concentrations. NO levels in LPS-stimulated RAW 264.7 cells exposed to 100 µg/ml of water, methanol, ethanol, or acetone extract were determined to be 91.1±2.7%, 81.3±1.3%, 24.0±4.6%, and 40.9±3.3%, respectively, relative to the untreated control. PGE2 levels following exposure to 100 µg/ml of the water, methanol, ethanol, or acetone extracts were determined to be 80.5±8.7%, 92.5±3.4%, 47.9±6.4%, and 64.6±2.8% of the control values. These results showed that only methanol, ethanol, and acetone extracts exerted an anti-inflammatory effect by ameliorating the production of the inflammatory mediators NO and PGE2. In contrast, equal concentration of water extract did not elicit anti-inflammatory effects. The water extract exhibited anti-inflammatory effects at 250 µ g/ml. In addition, PGE2 was dramatically reduced (82.5%) following treatment with 250 µg/ml of the water extract. The 250 µg/ml concentration in the acetone extract and water extract was reduced dramatically than the ethanol extract. These results indicates that the water and acetone extract was more related to pain. In particular, levels of NO were dramatically reduced (76.0%) compared to those of PGE2 and other inflammatory factors following incubation of RAW 264.7 cells with 100 µg/ml of the ethanol extract. Based on the comparison of the 4 Cynanchi wilfordii Radix extracts, the highest anti- inflammatory effects were observed with the ethanol extract.
The effect of Cynanchi wilfordii Radix extracts on the production of IL-1β, IL-6, IL-10, and TNF-α were evaluated in LPS-stimulated RAW 264.7 cells. As shown in Fig. 3, production of IL-1β, IL-6, IL-10, and TNF-α was dramatically diminished following treatment with the extracts. Treatment with ethanol extract in particular was observed to reduce IL-1β and IL-6 production to lower levels than treatments with the water, methanol, and acetone extracts. However, no inhibition of IL-10 production was observed following incubation with the water extract and no inhibition of TNF-α was observed following treatment with the water, methanol, or acetone extracts. TNF-α production was not significantly reduced, compared to other cytokines. Incubation with the highest concentration of the ethanol extract (250 µg/ml) was the only treatment that inhibited TNF-α.
Expression of iNOS and COX-2 enzymes was evaluated in murine macrophage RAW 264.7 cells using immunoblotting. As shown in Fig. 4, the ethanol extract was found to inhibit the expression levels of iNOS and COX-2 proteins in a concentration-dependent manner, with highest inhibitory effects observed following treatment with the 100 µg/ml concentration. Methanol and acetone extracts inhibited the protein expression levels of iNOS and COX-2 in a concentration-dependent manner, with highest inhibitory effects observed at 250 µg/ml. Conversely, water extract inhibited the protein expression levels of COX-2, with highest inhibition observed at 250 µg/ml.
The antioxidant activity of polyphenols was rapidly evaluated. As shown in Table 2, DPPH radical scavenging activity of Cynanchi wilfordii Radix extracts significantly increased with increasing extract concentration. Relatively high DPPH radical scavenging activities were found in water, methanol, ethanol, and acetone extracts (56.8%, 85.2%, 85.3%, and 83.2%, respectively) at 10.0 mg/ml. The highest DPPH radical scavenging activity (91.0%) was observed in the ethanol extract.
As shown in Table 3, ethanol extract elicited relatively higher anti-microbial activity against B. cereus and B. anthracis (12.3 mm and 12.7 mm, respectively) than the other extracts. However, no anti-microbial activity was observed against E. coli O157:H7, S. typhimurium, and V. cholerae O139. The disc diffusion values of the ethanol extract at 50 mg/ml concentration demonstrated its effectiveness against the bacteria involved in food poisoning. In contrast, the water, methanol, and acetone extracts were observed not to be effective in inhibiting bacteria involved in food poisoning. The MIC value of the ethanol extract was 6.25 mg/ml against B. cereus and B. anthracis (Table 4).
Cynanchi wilfordii Radix has been used in Korean Oriental medicine for the prevention and treatment of various diseases [2]. In recent years, a number of medical herbs are increasingly used all over the world to promote health through reinforcing immunocompetence. Cynanchi wilfordii Radix is known for its medical uses but is also recognized to be among the foods with health benefits. It is perceived to be as an important agent in alternative medicine throughout the world. Commonly used medical agents comprise chemical substances, and have a high incidence of side effects. In comparison, medicines composed of natural substances are less likely to cause side effects. In this study, the polyphenols of the medicinal plant Cynanchi wilfordii Radix were evaluated for their anti-inflammatory, anti-oxidant, and anti-microbial activities.
Phenolic compounds, especially phenolic acids, are present in vegetables, fruits, wines, juices, and tea. Recently, they have received much attention since a number of epidemiological studies suggest that consumption of polyphenol-rich foods and beverages may be protective against several chronic diseases, including heart disease, atherosclerosis, and cancer [24,25,26,27]. Therefore, the amount of polyphenol was measured in extracts of Cynanchi wilfordii Radix, with the ethanol extract determined to have the highest TPC, measured at 19.8±0.4 µg GAE/g.
Inflammatory reaction results in the generation of NO and PG. NO is involved in the pathogenesis of a variety of diseases, including inflammatory bowel disease [28], multiple sclerosis [29], rheumatoid arthritis [30], bronchitis [31], and gastritis [32]. In addition, pro-inflammatory cytokines and mediators regulate the functional activity of immune cells and are involved in the pathogenesis of a host of inflammatory diseases and hemorrhagic shock, including septic shock, ulcerative colitis, and atherosclerosis [33,34,35,36]. The results of this study show that ethanol extracts of Cynanchi wilfordii Radix exhibited the highest inhibitory effect on LPS-induced NO, PGE2 and cytokine production. Levels of NO and PGE2 were reduced, followed by a reduction in expression of the key proteins involved in their synthesis, iNOS and COX-2, respectively. Among the evaluated extracts, the ethanol extract was found to dramatically reduce inflammation in LPS-stimulated RAW 264.7 cells. Also, the cytotoxicity of the Cynanchi wilfordii Radix extracts were not obvious after 24 h incubation. Traditional plant-derived medicines are usually considered to be less toxic with fewer side effects than chemical substances [37,38]. Thus, Cynanchi wilfordii Radix ethanol extract that suppress or inhibit the expression of these inflammation-associated genes can be considered to have therapeutic effects in the prevention of inflammatory reactions and diseases. Therefore, Cynanchi wilfordii Radix may be effective in the prevention and treatment of inflammation induced by a bacterial pathogen or viral infection in RAW 264.7 cells.
Overproduction of reactive oxygen species can occur due to oxidative stress brought about by imbalances between the antioxidant defense system of the body and increased free radical formation. Oxidative stress has been linked with the progression of Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and DNA damage [39,40]. The highest DPPH scavenging activity was found in the Cynanchi wilfordii Radix ethanol extract, approaching 85.3% of the activity of α-tocopherol. Therefore, components of the Cynanchi wilfordii Radix ethanol extract may be able to improve cellular defenses and help to prevent oxidative damage to cellular components.
In addition, based on the evaluation of anti-bacterial activity against bacterial strains involved in food poisoning, ethanol extract of Cynanchi wilfordii Radix was observed to be effective against Bacillus cereus and Bacillus anthracis. These results indicate that polyphenols present in Cynanchi wilfordii Radix ethanol extract have anti-inflammatory, antioxidant, and anti-bacterial properties. The findings of the present study clearly indicate that the ethanol extract of Cynanchi wilfordii Radix is a candidate for clinical use in treatment and prevention of food-borne disease and gastroenteritis.
ACKNOWLEDGEMENTS
This research was supported by the Biomedical Science Scholarship Grants, Department of Medicine, Chung-Ang University in 2012.
References
1. Lamidi M, DiGiorgio C, Delmas F, Favel A, Eyele Mve-Mba C, Rondi ML, Ollivier E, Nze-Ekekang L, Balansard G. In vitro cytotoxic, antileishmanial and antifungal activities of ethnopharmacologically selected Gabonese plants. J Ethnopharmacol. 2005; 102:185–190. PMID: 16046090.

2. Han DS. Pharmacoligical studies on Ha-Su-O (Polygoni Radix). Korean J Pharmacogn. 1973; 4:83–94.
3. Deepak D, Srivastav S, Khare A. Pregnane glycosides. Fortschr Chem Org Naturst. 1997; 71:169–325. PMID: 9250024.
4. Kaneda N, Chai H, Pezzuto JM, Kinghorn AD, Farnsworth NR, Tuchinda P, Udchachon J, Santisuk T, Reutrakul V. Cytotoxic activity of cardenolides from Beaumontia brevituba stems. Planta Med. 1992; 58:429–431. PMID: 1470666.
5. Seo YW, Kim HH, Ko H. Effect of aqueous extract of polygoni multiflori radix on hypertension and arterial contraction in animal models. Korean J Orient Physiol Pathol. 2008; 22:593–599.
6. Shin MK. A Comparative study on the effects of polygoni radix and cynanchi radix on rat livers intoxicated with carbon tetrachloride. Korean J Pharmacogn. 1985; 16:81–92.
7. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795. PMID: 12360216.

8. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–29. PMID: 12495721.

9. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258. PMID: 15691589.

10. Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005; 23:787–819. PMID: 15771586.

11. Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989; 7:645–651. PMID: 2695408.

12. Morrissey PA, O'Brien NM. Dietary antioxidants in health and disease. Int Dairy J. 1998; 8:463–472.

13. Ehling-Schulz M, Fricker M, Scherer S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res. 2004; 48:479–487. PMID: 15538709.

14. Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. 2003; 41:3441–3444. PMID: 12843116.
15. Oh BK, Kim YK, Park KW, Lee WH, Choi JW. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens Bioelectron. 2004; 19:1497–1504. PMID: 15093222.

16. Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998; 11:450–479. PMID: 9665978.
17. Singh N, Singh RK, Bhunia AK, Stroshine RL. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing escherichia coli O157: H7 on lettuce and baby carrots. Lebensm-Wiss Techno. 2002; 35:720–729.
18. Ghelardi E, Celandroni F, Salvetti S, Barsotti C, Baggiani A, Senesi S. Identification and characterization of toxigenic Bacillus cereus isolates responsible for two food-poisoning outbreaks. FEMS Microbiol Lett. 2002; 208:129–134. PMID: 11934506.
19. Saile E, Koehler TM. Control of anthrax toxin gene expression by the transition state regulator abrB. J Bacteriol. 2002; 184:370–380. PMID: 11751813.
20. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Working Group on Civilian Biodefense. Anthrax as a biological weapon: medical and public health management. JAMA. 1999; 281:1735–1745. PMID: 10328075.
21. Folin O, Denis W. On phosphotungastic-phosphomolybdic compounds as color ragents. J Biol Chem. 1912; 12:239–243.
22. Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988; 36:2090–2097. PMID: 3240445.

23. LCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eighth edition. Clinical and Laboratory Standards Institute;2009.
24. Vinson JA, Hao Y, Su XH, Zubik L. Phenol antioxidant quantity and quality in foods: Vegetables. J Agr Food Chem. 1998; 46:3630–3634.

25. Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001; 49:5315–5321. PMID: 11714322.

26. Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis. 2001; 156:67–72. PMID: 11368998.

27. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71:1698S–1702S. PMID: 10837321.

28. Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005; 115:3057–3071. PMID: 16239967.
29. Klotz L, Schmidt M, Giese T, Sastre M, Knolle P, Klockgether T, Heneka MT. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005; 175:4948–4955. PMID: 16210596.
30. Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, Gesinde M, Taylor J, Markham AF, Emery P, van Laar JM, Isaacs JD. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002; 100:4550–4556. PMID: 12393721.

31. Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002; 26:152–159. PMID: 11751215.

32. Sakagami T, Vella J, Dixon MF, O'Rourke J, Radcliff F, Sutton P, Shimoyama T, Beagley K, Lee A. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect Immun. 1997; 65:3310–3316. PMID: 9234792.

33. Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999; 34:66–74. PMID: 10204613.

34. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533. PMID: 12876555.

35. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83:1505S–1519S. PMID: 16841861.

36. Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006; 177:4514–4520. PMID: 16982888.

37. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the Prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011; 2011:680354. PMID: 19596745.

38. Sergent T, Vanderstraeten J, Winand J, Beguin P, Schneider YJ. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012; 135:68–73.

39. Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004; 3:205–214. PMID: 15031734.

40. Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001; 122:497–506. PMID: 11570956.

Fig. 1
The viability of RAW 264.7 cells following treatment with a range of concentrations of Cynanchi wilfordii Radix extracts. Cells were treated for 24 h with 0, 1, 5, 10, 50, 100, or 250 µg/ml of Cynanchi wilfordii Radix extracts. Control cells were treated with media only. The results are expressed as means±SE from 3 independent experiments.
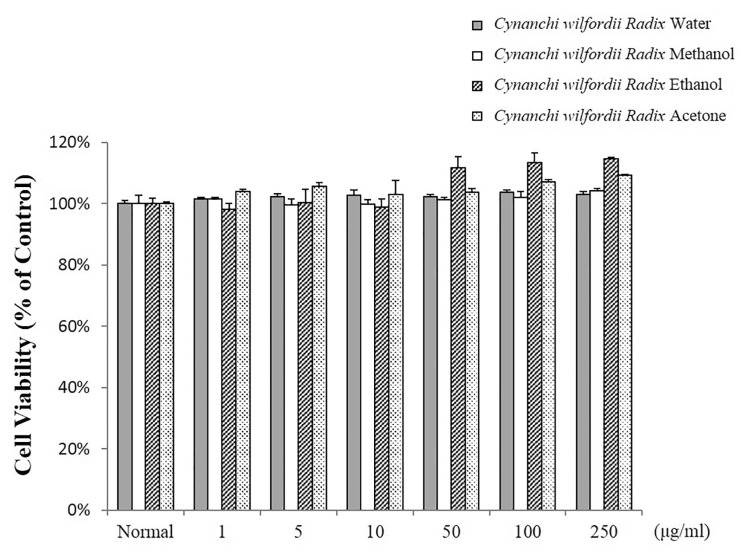
Fig. 2
Suppression of LPS-induced production of NO and PGE2 in RAW264.7 cells by Cynanchi wilfordii Radix extracts. Cells were treated for 18 h with 0, 1, 5, 10, 50, 100, or 250 µg/ml of Cynanchi wilfordii Radix extracts in the presence of 0.1 µg/ml LPS. Control cells were treated with media only. The results are expressed as means ± SE from 3 independent experiments. *p<0.01 indicates a significant difference compared to LPS-treated cells. A: NO, B: PGE2.
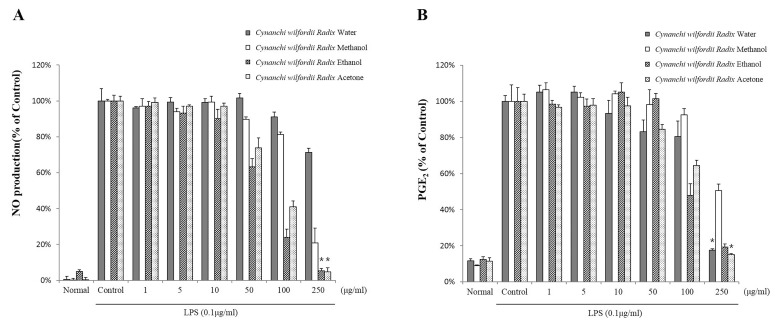
Fig. 3
Suppression of LPS-induced production of cytokines IL-1β, IL-6, IL-10, TNF-α in RAW264.7 cells by Cynanchi wilfordii Radix extracts. RAW 264.7 cells were treated for 18 h with 0, 1, 5, 10, 50, 100, or 250 µg/ml of Cynanchi wilfordii Radix extracts in the presence of 0.1 µg/ml LPS. Control cells were treated with media only. The cell culture media were subsequently collected. The results are expressed as means±SE from 3 independent experiments. *p<0.01 indicates significant difference compared to LPS-treated cells. A: IL-1β, B: IL-6, C: IL-10, D: TNF-α.
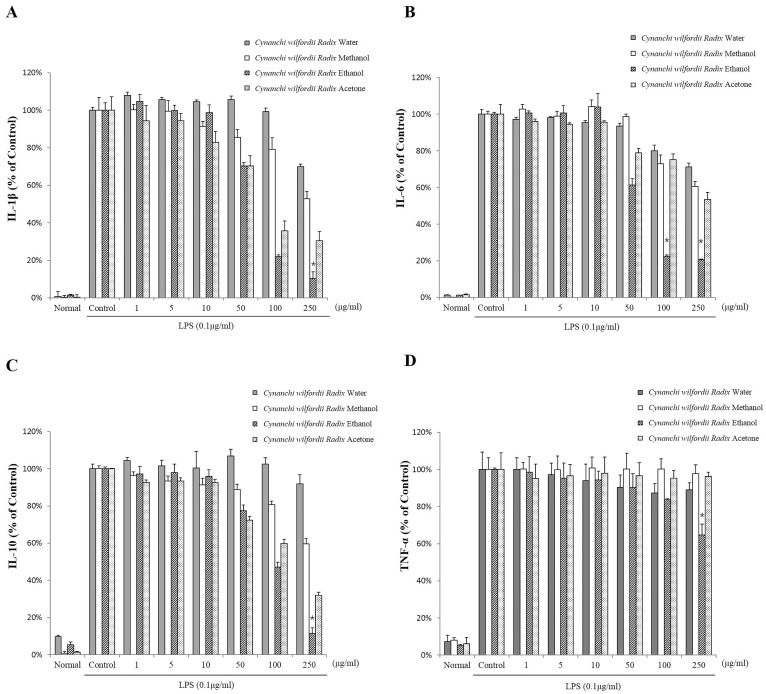
Fig. 4
Effects of Cynanchi wilfordii Radix extracts on LPS-induced changes in iNOS and COX-2 protein expression. Inhibitory effect of Cynanchi wilfordii Radix extracts on LPS induction of iNOS and COX-2 protein expression was evaluated in RAW264.7 macrophage cells. Cells were treated for 24 h with 0, 1, 5, 10, 50, 100, or 250 µg/ml of different Cynanchi wilfordii Radix extracts. Electrophoresis was performed on cell lysates, and the expression levels of iNOS and COX-2 were detected using specific antibodies. β-actin was used as an internal control for western blot analysis. A: water extract, B: methanol extract, C: ethanol extract, D: acetone extract.
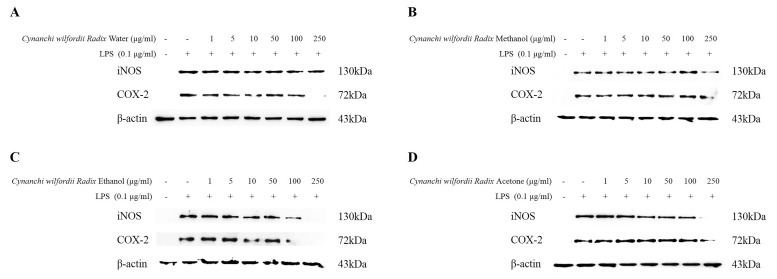
Table 2
2,2-Diphenyl-1-picrylhydrazyl hydrate (DPPH) radical scavenging activity of Cynanchi wilfordii Radix extracts, expressed as percentage of control*




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download