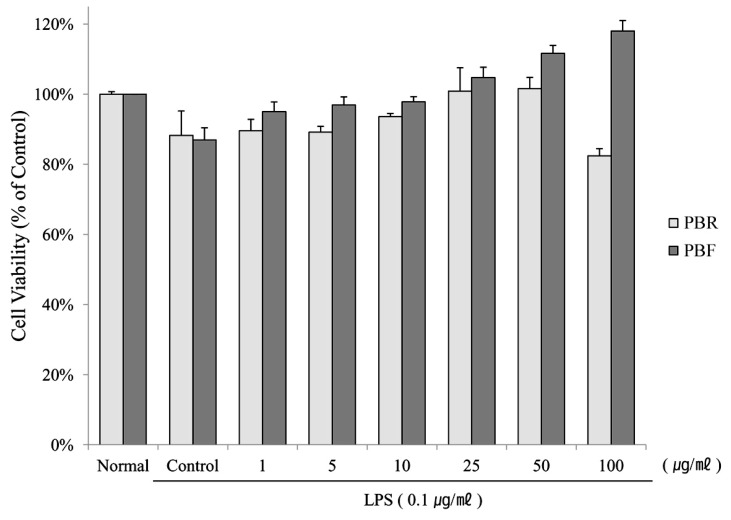
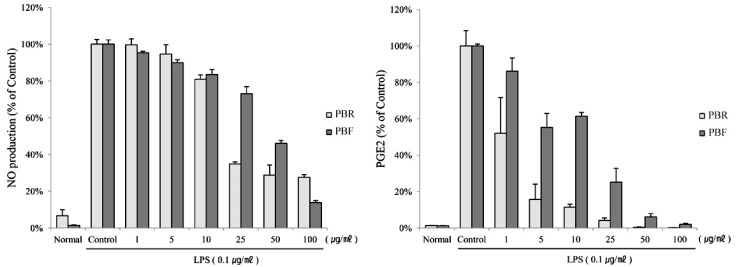
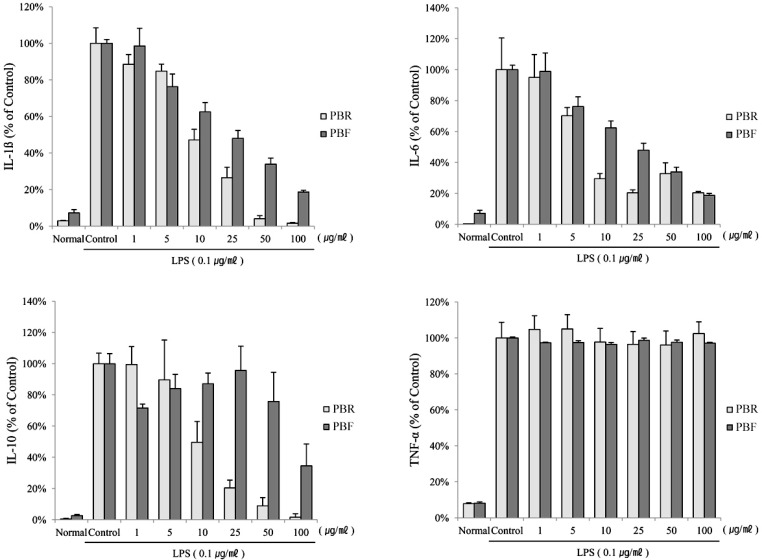
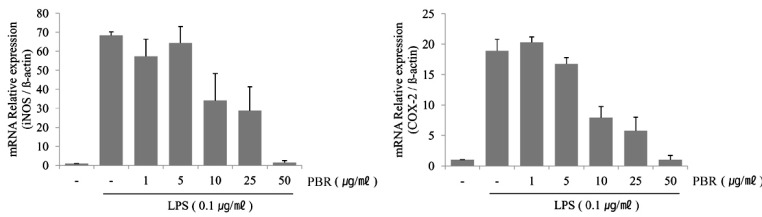
1. Kim EJ, Lee YJ, Shin HK, Park JH. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition. 2005; 21:1141–1148. PMID:
16308138.

2. Moon GS. Constituents and Uses of Medicinal Herbs. 1991. Seoul: Ilwolbooks.
3. Heo J. Donguibogam. 1994. Seoul: Yeogang Publishing Co..
4. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258. PMID:
15691589.

5. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795. PMID:
12360216.

6. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–29. PMID:
12495721.

7. Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005; 23:787–819. PMID:
15771586.

8. Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995; 13:437–457. PMID:
7542010.

9. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002; 23:144–150. PMID:
11864843.

10. Smith WL, Meade EA, DeWitt DL. Pharmacology of prostaglandin endoperoxide synthase isozymes-1 and -2. Ann N Y Acad Sci. 1994; 714:136–142. PMID:
8017762.
11. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2:907–916. PMID:
11577346.

12. Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995; 35:655–677. PMID:
7598511.

13. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998; 336:1–17. PMID:
9806879.

14. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:1–12. PMID:
19035777.

15. Moellering RC Jr. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008; 46:1032–1037. PMID:
18444820.
16. Esterly JS, Griffith M, Qi C, Malczynski M, Postelnick MJ, Scheetz MH. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother. 2011; 55:4844–4849. PMID:
21825287.

17. Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Nanas S, Markaki V, Vrettou C, Paniara O, Giamarellou H, Roussos C. Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: risk factors, clinical features and outcomes. Infection. 2010; 38:173–180. PMID:
20224962.

18. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999; 281:1735–1745. PMID:
10328075.
19. Yang HM, Oh SM, Lim SS, Shin HK, Oh YS, Kim JK. Anti-inflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother Res. 2008; 22:102–107. PMID:
17724764.

20. Quave CL, Estévez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, Smeltzer MS. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One. 2012; 7:e28737. PMID:
22242149.

21. Pan MH, Lai CS, Wang YJ, Ho CT. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem Pharmacol. 2006; 72:1293–1303. PMID:
16949556.

22. Kim SJ. Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. J Periodontal Implant Sci. 2011; 41:157–163. PMID:
21811692.
23. Lee JW, Do JH. Determination of total phenolic compounds from the fruit and Rubus coreanum and antioxidative activity. J Korean Soc Food Sci Nutr. 2000; 29:943–947.
24. Yoon I, Wee JH, Moon JH, Ahn TH, Park KH. Isolation and identification of quercetin with antioxidative activity from the fruits of Rubus coreanum Miquel. Korean J Food Sci Technol. 2003; 35:499–502.
25. Jeon SK, Lee JW, Lee IS. Effect of antioxidant activity and induction of DNA damage on human gastric cancer cell by Rubus coreanus Miquel. J Life Sci. 2007; 17:1723–1728.

26. Cho YJ, Chun SS, Kwon HJ, Kim JH, Yoon SJ, Lee KH. Comparison of physiological activities between hot-water and ethanol extracts of Bokbunja (Rubus coreanum F.). J Korean Soc Food Sci Nutr. 2005; 34:790–796.
27. Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ, Park HJ. Antinociceptive and anti-inflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull. 2003; 26:1436–1441. PMID:
14519951.

28. Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002; 8:321–331. PMID:
12084099.

29. Beck-Sagué CM, Jarvis WR, Brook JH, Culver DH, Potts A, Gay E, Shotts BW, Hill B, Anderson RL, Weinstein MP. Epidemic bacteremia due to Acinetobacter baumannii in five intensive care units. Am J Epidemiol. 1990; 132:723–733. PMID:
2403113.
30. Smolyakov R, Borer A, Riesenberg K, Schlaeffer F, Alkan M, Porath A, Rimar D, Almog Y, Gilad J. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hosp Infect. 2003; 54:32–38. PMID:
12767844.