Abstract
High glucose leads to physio/pathological alterations in diabetes patients. We investigated collagen production in human gingival cells that were cultured in high concentrations of glucose. Collagen synthesis and secretion were increased when the cells were exposed to high concentrations of glucose. We examined endoplasmic reticulum (ER) stress response because glucose metabolism is related to ER functional status. An ER stress response including the expression of glucose regulated protein 78 (GRP78), C/EBP homologous protein (CHOP), inositol requiring enzyme alpha (IRE-1α) and phosphoreukaryotic initiation factor alpha (p-eIF-2α) was activated in the presence of high glucose. Activating transcription factor 4 (ATF-4), a downstream protein of p-eIF-2α as well as a transcription factor for collagen, was also phosphorylated and translocalized into the nucleus. The chemical chaperone 4-PBA inhibited the ER stress response and ATF-4 phosphorylation as well as nuclear translocation. Our results suggest that high concentrations of glucose-induced collagen are linked to ER stress and the associated phosphorylation and nuclear translocation of ATF-4.
Type 2 diabetes is characterized by abnormally elevated blood glucose levels. Prolonged or chronic hyperglycemia causes or contributes to detrimental effects such as diabetic complications and exacerbation in various tissues, such as the kidney, eye, peripheral nerves, pancreatic β-cells and periodontal/gingival environments. Prolonged hyperglycemia may have detrimental effects on protein secretion/biosynthesis [1,2] and induces endoplasmic reticulum (ER) stress [3,4] which occurs when either the protein folding capacity of the ER is not sufficient to meet protein folding demands or excess misfolded or aggregated proteins accumulate. Such conditions activate the unfolded protein response (UPR), which transiently reduces new protein synthesis and increases folding capacity and degradation of terminally misfolded proteins [5]. ER stress affects other cells such as pancreatic β-cells, which must regulate their own chaperone capacity to meet normal insulin biosynthetic demands. Insulin translation is regulated in β-cells via eukaryotic translation initiation factor 2 alpha (eIF2α) phosphorylation by the PKR-like kinase (PERK) pathway [6] which is critical to β-cell function. In diabetes mellitus, high glucose levels cause coordinated alterations in cytokines, growth factors and hormones; this leads to excessive deposition of extracellular matrix (ECM), resulting in tissue injury [7]. While mesothelial cells have the capacity to produce a variety of matrix proteins, including collagen in the basal state [8], high glucose stimulates fibronectin mRNA expression in mesothelial cells [9], suggesting the involvement of fibrosis. Intense tissue remodeling and fibrosis also occur in the periodontal environment when exposed to high glucose levels [10]. The fibrotic process is progressive and facilitates inflammation. The process of fibrosis can be severe in the periodontal environment when diabetic complications occur [11]. The possible consequences and disease contributions of prolonged ER stress due to chronic hyperglycemia are not well studied in the periodontal field. Despite the key roles of gingival cells in periodontal inflammation and other pathologies, there have not been many studies of diabetes-associated gingival alterations and other physio-pathologic changes, and the mechanistic disease aspects are poorly understood.
Chemical chaperones are low molecular weight compounds known to improve ER folding capacity. They also serve as a type of 'quality control system', recognizing, retaining and targeting misfolded proteins for their eventual degradation [12]. One chemical chaperone, 4-phenylbutyric acid (4-PBA), is a nontoxic butyrate analog that was approved for clinical use as an ammonia scavenger in subjects with urea cycle disorders [13]. Since a number of studies have suggested a potential role of chemical chaperones in the treatment of ER stress-related disease such as Alzheimer's disease, prion disease, fibrosis and diabetes [14-16], the application of chemical chaperone into periodontal inflammation and diabetes-associated pathological system seems to be scientifically meaningful.
In this study, we examined the effects of prolonged high glucose levels on activation of the ER stress pathway, as well as fibrosis-associated in vitro collagen production and secretion in human gingival cells. In addition, the chemical chaperone, 4-PBA was used in the in vitro model in order to show the role of ER stress in high concentration of glucose-exposed collagen synthesis and secretion.
Antibodies against pro-collagen, collagen, GRP78, CHOP and actin were purchased from Cell Signaling Technologies (Beverly, MA, USA). Antibodies against p-eIF2α, eIF-2α, IRE-1α, p-ATF-4, ATF-4, PARP and pro-collagen I were obtained from Santacruz (St. Louis, MO, USA). Antibody against collagen I was obtained from Abcam (Cambridge, MA, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin, and other tissue culture reagents were supplied by Life Technologies, Inc. (Grand Island, NY, USA). Bicinchoninic acid protein assay reagents were obtained from Pierce Biotechnology (Rockford, CA, USA). All other chemicals were at least of analytical grade and were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Human gingival fibroblasts from ScienCell Research Laboratories were isolated from human gingiva and were cultured in DMEM supplemented with 10% FBS, 20 mM 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid, 100 mg/ml streptomycin, and 100 units/ml penicillin.
For nucleus extraction, we followed a modified protocol from a previously reported study [17]. After harvesting cells, they were lysed with lysis buffer (1 M HEPES, 3 M KCl, 0.25 M EDTA, 10% NP40, H2O, 1 M DTT containing protease inhibitors (aprotinin, leupeptin, pepstatin A, antipain, phenylmethylsulfonyl fluoride)). The cells were pelleted by centrifugation at 14 K rpm for 10 min at 4C. Then, we collected the pellet as a nucleus fraction. For the extraction of nuclear protein, the extraction buffer (1 M HEPES, 3 M NaCl, 0.25 M EDTA, H2O, 1 M DTT containing protease inhibitors (aprotinin, leupeptin, pepstatin A, antipain, phenylmethylsulfonyl fluoride)) was added to the nucleus fraction. After incubation for 10 min at 4C, supernatant was separated from pellet containing nuclear protein.
Cells were treated with drugs for the indicated times and harvested by washing twice with ice-cold PBS on ice. For preparation of whole-cell lysates, cells were lysed on ice by adding RIPA lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP40, 1 mM ethylenediaminetetraacetic acid, 0.1% sodium dodecyl sulfate] plus protease inhibitor cocktail set III and phosphatase inhibitor cocktail set II (EMD Biosciences, La Jolla, CA, USA) directly onto the cells. Cell lysates were then transferred to microtubes and incubated for 30 min on ice, followed by centrifugation at 12,000 rpm for 10 min at 4℃, and supernatants were collected to obtain protein extracts. Protein extracts were then added to the sample buffer, boiled in a water bath for 5 min and stored at 20℃ until use. Ten micrograms of extracted proteins were run on a polyacrylamide gel and transferred to a nitrocellulose membrane, which was then blocked with Tris-buffered saline solution containing 0.05% Tween-20 for 30 min at room temperature. The blots were probed overnight at 4℃ with the relevant antibodies, washed, and probed again with species-specific secondary antibodies coupled to horseradish peroxidase (GE Healthcare, Piscataway, NJ, USA). After washing, the immunoreactive proteins were detected by chemiluminescence (GE Healthcare) and exposure of the membranes to Hyperfilm ECL. The intensities of bands were quantified using Scion-Image for Windows (Scion Corporation, Frederick, MA, USA).
The levels of collagen in the media from gingival cells were determined by a SIRCOL collagen assay (Biocolor Ltd., UK) according to manufacturer's instructions. Briefly, medium was incubated with Sirius red dye and absorbance was determined at 555 nm with a spectrophotometer (Infinite M200, Tecan, Austria). The amount of soluble collagen was expressed in mg/g of protein in the medium.
To investigate the role of glucose in gingival fibroblast physiology, secreted collagen was measured in the medium from cells cultured with 5, 10, 20, 30, or 40 mM glucose. Cells were pre-cultured in media with low concentrations of glucose (5 mM). We found that secreted soluble collagen increased in a glucose concentration-dependent manner in human gingival cells (Fig. 1A). Cell lysates from the same conditions were used for immunoblotting. Pro-collagen was highly expressed at high concentrations of glucose (20, 30 or 40 mM; Fig. 1B), suggesting that high glucose activated synthesis of pro-collagen. Active collagen and collagen 1 showed similar increases in expression (Fig. 1B, lower). The level of collagen 1 in the medium also increased in a glucose concentration-dependent manner (Fig. 1C).
When the cells were cultured in 5 or 40 mM glucose-contained medium, soluble collagen was increased in the medium as the incubation period increased (Fig. 2A). Inside, the expressions of pro-collagen 1 and collagen 1 were also increased in an incubation time-dependent manner (Fig. 2B). Secreted collagen 1 was increased in a time-dependent manner (Fig. 2C).
Recent studies have examined the effects of Type II diabetes and ER stress [3,4,18]. Pancreatic beta cells and liver cells have frequently been studied to understand the role of glucose on ER stress responses. Here, we aimed to also examine how high glucose could regulate collagen expression. We first analyzed the ER stress response including induction of eIF-2α and its downstream transcription factor, ATF-4 in cells cultured in 5, 10, 20, 30 or 40 mM glucose. As expected, the expression of GRP78, CHOP, p-eIF2α, p-ATF-4 and IRE-1α increased with increasing glucose concentrations (Fig. 3A). The quantification ratio is shown at the right panel of Fig. 3A. Phospho-eIF-2α and phospho-ATF-4 levels were compared to total eIF-2α and ATF-4 expression, respectively. Other proteins including GRP78 were compared to β-actin. Similarly, GRP78, CHOP, p-eIF2α, p-ATF-4 and IRE-1α were also highly expressed with increased incubation periods as glucose increased (Fig. 3B), suggesting that high glucose can induce ER stress in gingival fibroblasts. The quantification ratio is shown at the right panel of Fig. 3B. Then, the nuclear translocation of ATF4, a transcription factor for collagen as well as a downstream target of eIF-2α, was examined using isolated nuclear fractions. The nuclear expression of ATF-4 was increased in a glucose concentration-dependent and a time-dependent manner (Fig. 3C). The expression of PARP was shown as a loading control.
We hypothesized that relieving ER stress in the cells would inhibit collagen accumulation in the medium. 4-Phenylbutyric acid (4-PBA), a small chemical chaperone, has been shown to reduce ER stress both in vivo and in vitro [19]. When the cells were treated with 4-PBA, the expression levels of ER stress response-associated proteins, GRP78, CHOP, p-eIE-2α and IRE-1α as well as pro-collagen 1 and collagen 1 were significantly reduced (Fig. 4A). A quantification analysis was also performed (right panel, Fig. 4A). The phosphorylation of ATF-4, a transcription factor for collagen as well as a downstream target of eIF-2α, was inhibited by 4-PBA. Fig. 4B also shows that the nuclear translocation of ATF-4 was reduced in the presence of 4-PBA. Collagen secreted into the medium was also reduced in the presence of 4-PBA (Fig. 4C), and 4-PBA blocked the presence of collagen 1 in the medium (Fig. 4D). These results suggest that the action of 4-PBA may be linked to collagen synthesis or secretion. Thus, the ER stress response and its related transcription factor ATF-4 may be involved in glucose-dependent regulation of collagen synthesis or secretion.
Periodontal disease is highly prevalent and understanding its causative and progression factors are important issues in public health [11]. The aim of this study was to characterize gingival fibrosis as a phenomenon observed in diabetes-associated periodontal disease. Collagen synthesis and secretion were enhanced when gingival cells were cultured in the presence of high glucose. We hypothesized that the main organelle involved in protein folding and secretion, the ER, may be involved in collagen synthesis and secretion. We investigated the effect of the ER stress regulator 4-PBA on collagen regulation in gingival cells and confirmed that the ER stress response is involved in this system. 4-PBA reversed the collagen accumulation in extracellular medium suggesting that 4-PBA was a regulator of collagen accumulation in gingival fibroblasts.
Accumulation of ECM components is not only a characteristic pathological finding in these diseases [20], but can also result from imbalances of synthesis and degradation of ECM components, including collagen, fibronectin and laminin. Although cell growth was slow in media with high concentrations of glucose (data not shown), the pro-form of collagen 1 was highly expressed along with the active form, collagen 1 (Fig. 1C). In addition to the synthesis of ECM components, the degradation of ECM by key proteolytic enzymes including matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) is also important. Among these, gelatinases (MMP-2 and -9) and TIMPs (TIMP-1 and -2) have been widely studied [21,22]. In this study, MMP-2 activity was decreased in the presence of high concentrations of glucose (data not shown). The reduced proteinase activity may also have affected the expression of secreted soluble collagen 1, contributing to the significantly increased overall expression pattern of collagen 1 (2C). A similar decrease in MMP-2 or MMP-9 gene expression has also been observed in human and animal models of diabetes [23,24]. This study suggests that high glucose, a representative characteristic of diabetes, increases extracellular collagen accumulation, presumably involving an imbalance of collagen synthesis and degradation.
ER stress responses were observed in human gingival cells when they were exposed to high levels of glucose (Fig. 3A, B). The ER stress protein PERK is a transmembrane serine/threonine protein kinase that contains an N-terminal ER luminal domain and a cytoplasmic C-terminal protein kinase domain [25,26]. PERK phosphorylates eIF2α [25] and phosphorylation of eIF2α attenuates initiation of translation of most transcripts while concurrently increasing translation of select mRNAs such as ATF4 transcription factor [27,28]. ATF4-dependent collagen synthesis has been documented in various cells [29,30]. In the presence of high glucose, ATF4 was highly phosphorylated (Fig. 3A, B) as well as the nuclear translocation of ATF4 was also increased in human gingival cells (Fig. 3C), suggesting that eIF-2α-linked ATF4-dependent collagen synthesis increased in cells cultured with high glucose concentrations. Although ATF4 expression was also known to be involved in cell death mechanisms linking the CHOP signal transduction pathway, the human gingival cells did not show any cell death pattern during culture periods longer than 5 days. In this study, the eIF2α-linked ATF4 phosphorylation and nuclear translocation are the physiological ER stress signaling factors linked to collagen synthesis and secretion, but not cell death.
A number of studies previously demonstrated that the low molecular weight fatty acid, 4-PBA, can act as a chemical chaperone, assisting with protein folding and thus relieving the cell of ER stress [31-33]. In this study, we showed that 4-PBA can attenuate the ER stress response as well as ATF-4 phosphorylation and nuclear translocation in addition to the resulting collagen synthesis and secretion (Fig. 4). We suggest that the 4-PBA-induced regulation of extracellular collagen accumulation includes ER stress regulation and inhibition of ATF-4 that leads to reduced collagen synthesis and secretion.
In conclusion, high glucose increased collagen synthesis and secretion, and involves the ER stress response and the associated activation of ATF-4 in human gingival cells. This study also suggests the possibility of using 4-PBA as a therapeutic/preventive agent for collagen accumulation in gingival fibrosis.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084272).
References
1. Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002; 45:309–326. PMID: 11914736.

2. Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006; 136:873–876. PMID: 16549443.

3. Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, Won KC, Park JY, Lee KU, Choi HS, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008; 149:3832–3841. PMID: 18450959.

4. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006; 4:245–254. PMID: 16950141.

5. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529. PMID: 17565364.

6. Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic {beta}-cells. J Biol Chem. 2009; 284:5289–5298. PMID: 19103594.
7. Lee HB, Ha H. Experimental approaches to diabetic nephropathy. Kidney Int. 1997; 52(Suppl 60):S1–S2.
8. Perfumo F, Altieri P, Degl'Innocenti ML, Ghiggeri GM, Caridi G, Trivelli A, Gusmano R. Effects of peritoneal effluents on mesothelial cells in culture: cell proliferation and extracellular matrix regulation. Nephrol Dial Transplant. 1996; 11:1803–1809. PMID: 8918626.
9. Kumano K, Schiller B, Hjelle JT, Moran J. Effects of osmotic solutes on fibronectin mRNA expression in rat peritoneal mesothelial cells. Blood Purif. 1996; 14:165–169. PMID: 8785032.

10. Silva JA, Lorencini M, Reis JR, Carvalho HF, Cagnon VH, Stach-Machado DR. The influence of type I diabetes mellitus in periodontal disease induced changes of the gingival epithelium and connective tissue. Tissue Cell. 2008; 40:283–292. PMID: 18439638.

11. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001; 6:99–112. PMID: 11887478.

12. Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996; 1:109–115. PMID: 9222596.

13. Kajimura K, Takagi Y, Ueba N, Yamasaki K, Sakagami Y, Yokoyama H, Yoneda K. Protective effect of Astragali Radix by intraperitoneal injection against Japanese encephalitis virus infection in mice. Biol Pharm Bull. 1996; 19:855–859. PMID: 8799486.

14. Zhao JH, Liu HL, Lin HY, Huang CH, Fang HW, Chen SS, Ho Y, Tsai WB, Chen WY. Chemical chaperone and inhibitor discovery: potential treatments for protein conformational diseases. Perspect Medicin Chem. 2007; 1:39–48. PMID: 19812735.

15. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313:1137–1140. PMID: 16931765.

16. Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997; 100:2457–2465. PMID: 9366560.

17. Tanaka T, Tomaru Y, Nomura Y, Miura H, Suzuki M, Hayashizaki Y. Comprehensive search for HNF-1beta-regulated genes in mouse hepatoma cells perturbed by transcription regulatory factor-targeted RNAi. Nucleic Acids Res. 2004; 32:2740–2750. PMID: 15148361.
18. Rieusset J. Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int J Biochem Cell Biol. 2011; 43:1257–1262. PMID: 21605696.

19. Roque T, Boncoeur E, Saint-Criq V, Bonvin E, Clement A, Tabary O, Jacquot J. Proinflammatory effect of sodium 4-phenylbutyrate in deltaF508-cystic fibrosis transmembrane conductance regulator lung epithelial cells: involvement of extracellular signal-regulated protein kinase 1/2 and c-Jun-NH2-terminal kinase signaling. J Pharmacol Exp Ther. 2008; 326:949–956. PMID: 18574003.
20. Adler S. Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol. 1994; 5:1165–1172. PMID: 7873725.

21. Lee GH, Yan C, Shin SJ, Hong SC, Ahn T, Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, Kim DS, Chae SW, Kim HR, Chae HJ. BAX inhibitor-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium-hydrogen exchanger: the alteration of mitochondrial function. Oncogene. 2010; 29:2130–2141. PMID: 20118983.

22. Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J Cell Sci. 2011; 124:2220–2230. PMID: 21652634.

23. Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia. 1997; 40:1449–1454. PMID: 9447953.

24. McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002; 45:268–275. PMID: 11935159.

25. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999; 397:271–274. PMID: 9930704.

26. Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998; 18:7499–7509. PMID: 9819435.
27. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5:897–904. PMID: 10882126.

28. Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004; 101:11269–11274. PMID: 15277680.

29. Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009; 119:2807–2817. PMID: 19726872.

30. Disthabanchong S, Radinahamed P, Stitchantrakul W, Hongeng S, Rajatanavin R. Chronic metabolic acidosis alters osteoblast differentiation from human mesenchymal stem cells. Kidney Int. 2007; 71:201–209. PMID: 17183249.

31. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313:1137–1140. PMID: 16931765.

32. Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004; 66:899–908. PMID: 15226415.

33. Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol. 2000; 278:C259–C267. PMID: 10666020.
Fig. 1
Glucose increases the secretion of soluble collagen. Cells were exposed to the indicated concentrations of glucose for 1, 2, 3 or 4 days. (A) Cells were exposed to the indicated concentrations of glucose for 3 days. Soluble collagen was then analyzed in the media. (B) Cell lysates were used for immunoblotting with anti-pro-collagen 1 or collagen 1 antibody. (C) Immunoblotting using the medium from cells cultured with 40 mM glucose for the indicated periods. Anti-collagen 1 antibody immunoblot (upper) and a separate Coomassie blue-stained gel (lower) are shown. Data represent means±S.E. (n=3). *p<0.05, significantly different from 5 mM glucose-treated cells; NS, non-specific.
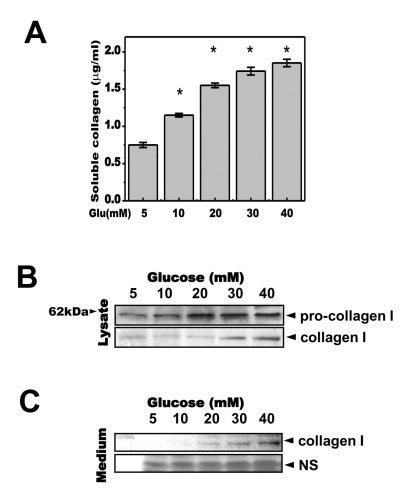
Fig. 2
High concentrations of glucose increased secretion of soluble collagen in a time-dependent manner. Cells were exposed to the indicated concentrations of glucose for 1, 2, 3 or 4 days. Soluble collagen was analyzed in the media (A). Cells were exposed to a concentration of 40 mM glucose for the same period. Cell lysates were then used for immunoblotting with anti-pro-collagen 1 or collagen 1 antibody (B). Immunoblotting using the medium from cells cultured with 40 mM glucose for the indicated periods with anti-collagen I antibody (C) and separate Coomassie blue staining (C, lower). Data represent means±S.E. (n=4). *p<0.05, significantly different from 5 mM glucose-exposed cells during each same period; NS, non-specific.
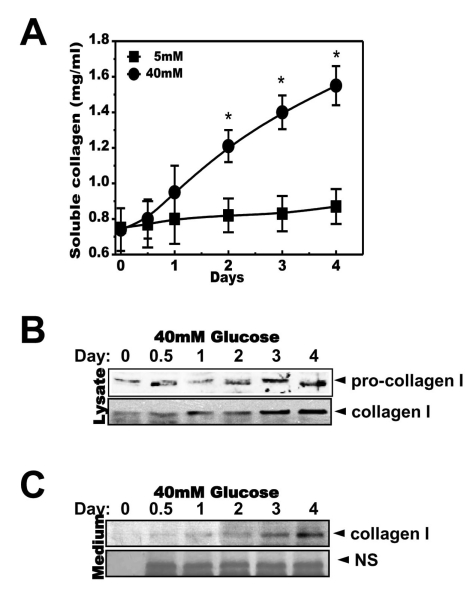
Fig. 3
Glucose induces the ER stress response. Cells were exposed to the indicated concentrations of glucose for 3 days. Immunoblotting was performed with anti-GRP78, CHOP, peIF-2α, eIF-2α, IRE-1α or β-actin antibodies (A). Quantification analysis was performed (right). Cells were exposed to 40 mM glucose for the indicated periods. Immunoblotting was performed with anti-GRP78, CHOP, p-eIF-2α, eIF-2α, IRE-1α or β-actin antibodies (B). Quantification was performed (right). After cells were exposed to the indicated concentrations of glucose for 3 days (C, upper) and separately cells were exposed to 40 mM glucose for the indicated periods (C, lower), nuclear fractions were obtained and immunoblotting was performed with anti-ATF-4 or PARP antibodies. Quantification was performed (right). Data represent means±S.E. (n=3). *p<0.05, significantly different from 5 mM glucose-exposed cells during each same period. #p<0.05, significantly different from 40 mM glucosetreated condition during 0 day.
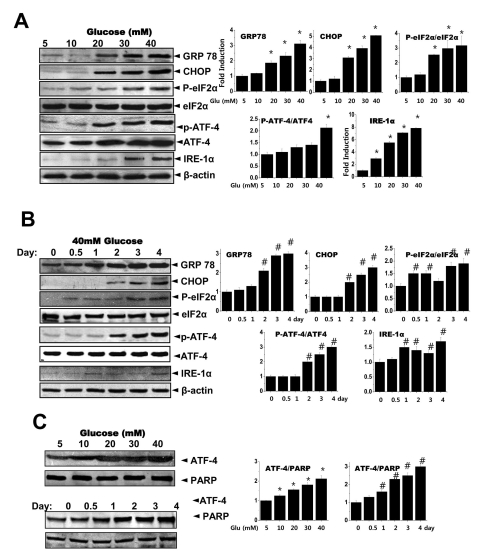
Fig. 4
The chemical chaperone, 4-PBA inhibits the ER stress response as well as collagen synthesis and secretion. Cells were exposed to the indicated concentrations of glucose with or without 4-PBA for 3 days. Immunoblotting was performed with anti-GRP78, CHOP, p-eIF-2α, eIF-2α, IRE-1α, pro-collagen I, collagen I or β-actin antibodies (A). Quantification was performed (right). Separately, cells were exposed to the indicated concentrations of glucose with or without 4-PBA for 3 days and nuclear fractions were obtained and immunoblotting was performed with either anti-ATF-4 or PARP antibodies (B). Quantification was performed (right). Soluble collagen was analyzed in media from the cells cultured with 5 or 40 mM glucose for 3 days (C). Immunoblotting was performed with the medium from the cells cultured with 40 mM glucose for 3 days, using anti-collagen I antibody (D) and separately stained with Coomassive blue dye (lower). Data represent means±S.E. (n=5). *p<0.05, significantly different from each indicated concentration of glucose-treated cells without 4-PBA. CBB; Coomassive blue staining.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download