Abstract
The effects of extremely low frequency electromagnetic fields (EMF) on intracellular Ca2+ mobilization and cellular function in RBL 2H3 cells were investigated. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not produce any cytotoxic effects in RBL 2H3 cells. Melittin, ionomycin and thapsigargin each dose-dependently increased the intracellular Ca2+ concentration. The increase of intracellular Ca2+ induced by these three agents was not affected by exposure to EMF (60 Hz, 1 mT) for 4 or 16 h in RBL 2H3 cells. To investigate the effect of EMF on exocytosis, we measured beta-hexosaminidase release in RBL 2H3 cells. Basal release of beta-hexosaminidase was 12.3±2.3% in RBL 2H3 cells. Exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h did not affect the basal or 1μM melittin-induced beta-hexosaminidase release in RBL 2H3 cells. This study suggests that exposure to EMF (60 Hz, 0.1 or 1 mT), which is the limit of occupational exposure, has no influence on intracellular Ca2+ mobilization and cellular function in RBL 2H3 cells.
References
1. Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979; 109:273–284.

2. Floderus B, Persson T, Stenlund C, Wennberg A, Ost A, Knave B. Occupational exposure to electromagnetic fields in relation to leukemia and brain tumors: a case-control study in Sweden. Cancer Causes Control. 1993; 4:465–476.

3. Matanoski GM, Elliott EA, Breysse PN, Lynberg MC. Leukemia in telephone linemen. Am J Epidemiol. 1993; 137:609–619.

4. Song HS, Kim HR, Ko MS, Jeong JM, Kim YH, Kim MC, Hwang YH, Sohn UD, Gimm YM, Myung SH, Sim SS. Effect of extremely low frequency electromagnetic fields (EMF) on phospholipase activity in the cultured cells. Korean J Physiol Pharmacol. 2010; 14:427–433.

5. Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984; 312:315–321.

6. Somlyo AV, Bond M, Somlyo AP, Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci USA. 1985; 82:5231–5235.

7. Nielsen OH, Bouchelouche PN, Berild D. Arachidonic acid and calcium metabolism in rnelittin stimulated neutrophils. Mediators Inflamm. 1992; 1:313–317.

8. Kahr H, Schindl R, Fritsch R, Heinze B, Hofbauer M, Hack ME, Mörtelmaier MA, Groschner K, Peng JB, Takanaga H, Hediger MA, Romanin C. CaT1 knock-down strategies fail to affect CRAC channels in mucosal-type mast cells. J Physiol. 2004; 557:121–132.

9. Shukla N, Freeman N, Gadsdon P, Angelini GD, Jeremy JY. Thapsigargin inhibits angiogenesis in the rat isolated aorta: studies on the role of intracellular calcium pools. Cardiovasc Res. 2001; 49:681–689.

10. Woerdenbag HJ, Merfort I, Passreiter CM, Schmidt TJ, Willuhn G, van Uden W, Pras N, Kampinga HH, Konings AW. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994; 60:434–437.
11. Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004; 43:11701–11708.

12. Huang F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue K, Takano H, Yoshino S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int Immunopharmacol. 2008; 8:502–507.

13. Hemmerling J, Nell S, Kipp A, Schumann S, Deubel S, Haack M, Brigelius-Flohé R. alpha-Tocopherol enhances degranulation in RBL-2H3 mast cells. Mol Nutr Food Res. 2010; 54:652–660.
14. Ikeda K, Shinmura Y, Mizoe H, Yoshizawa H, Yoshida A, Kanao S, Sumitani H, Hasebe S, Motomura T, Yamakawa T, Mizuno F, Otaka Y, Hirose H. No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro. Bioelectromagnetics. 2003; 24:21–31.
15. Yan J, Dong L, Zhang B, Qi N. Effects of extremely low-frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagn Biol Med. 2010; 29:165–176.

16. Morabito C, Guarnieri S, Fanò G, Mariggiò MA. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2010; 26:947–958.

17. Gaetani R, Ledda M, Barile L, Chimenti I, De Carlo F, Forte E, Ionta V, Giuliani L, D'Emilia E, Frati G, Miraldi F, Pozzi D, Messina E, Grimaldi S, Giacomello A, Lisi A. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc Res. 2009; 82:411–420.

18. Bernabò N, Tettamanti E, Pistilli MG, Nardinocchi D, Berardinelli P, Mattioli M, Barboni B. Effects of 50 Hz extremely low frequency magnetic field on the morphology and function of boar spermatozoa capacitated in vitro. Theriogenology. 2007; 67:801–815.
19. Pilger A, Ivancsits S, Diem E, Steffens M, Kolb HA, Rüdiger HW. No effects of intermittent 50 Hz EMF on cytoplasmic free calcium and on the mitochondrial membrane potential in human diploid fibroblasts. Radiat Environ Biophys. 2004; 43:203–207.
20. Madec F, Billaudel B, Charlet de Sauvage R, Sartor P, Veyret B. Effects of ELF and static magnetic fields on calcium oscillations in islets of Langerhans. Bioelectrochemistry. 2003; 60:73–80.

21. Rajkovic V, Matavulj M, Johansson O. Combined exposure of peripubertal male rats to the endocrine-disrupting compound atrazine and power-frequency electromagnetic fields causes degranulation of cutaneous mast cells: a new toxic environmental hazard? Arch Environ Contam Toxicol. 2010; 59:334–341.

22. International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010; 99:818–836.
Fig. 1.
The effect of EMF on cell viability of RBL 2H3 cells. The cells were exposed to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h and viability was measured with MTT assay. Results are indicated in mean±S.D. from four separate experiments.
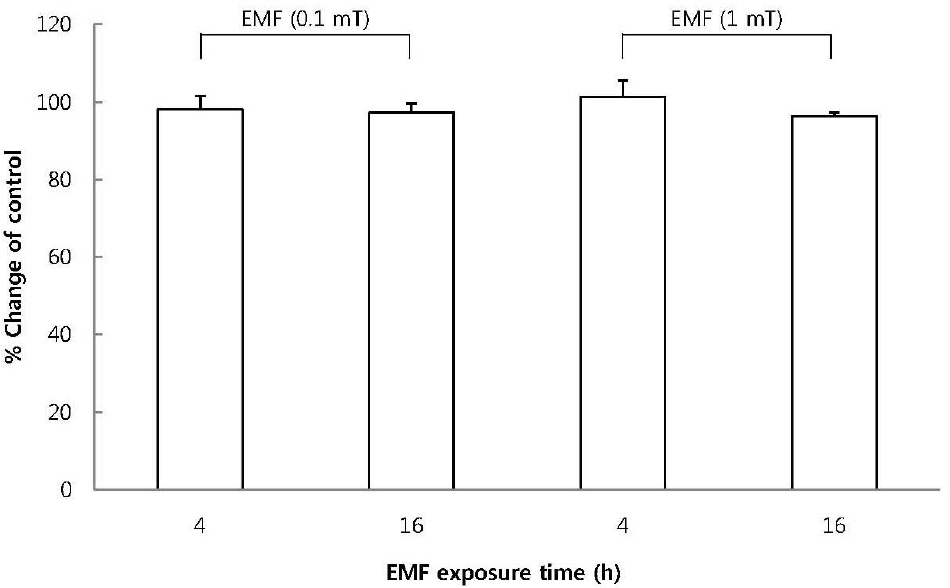
Fig. 2.
The effect of EMF on the intracellular Ca2+ mobilization induced by melittin in RBL 2H3 cells. Intracellular Ca2+ mobilization induced by melittin was measured with Quanta Master in Fura-2AM-loaded RBL 2H3 cells. Melittin dose-dependently increased intracellular Ca2+ mobilization (A) and 0.5μM melittin-induced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
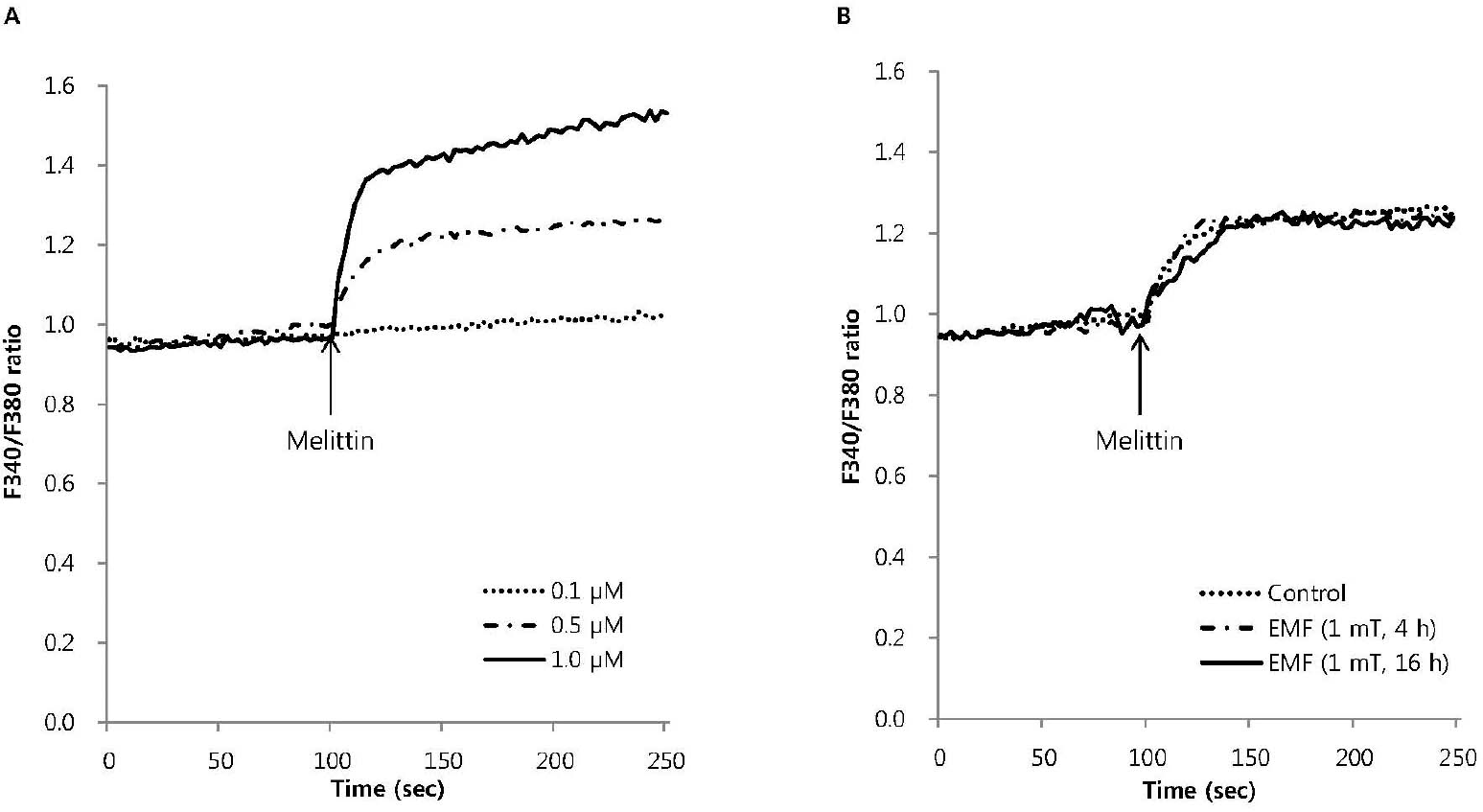
Fig. 3.
The effect of EMF on intracellular Ca2+ mobilization induced by ionomycin in RBL 2H3 cells. Ionomycin dose-dependently increased intracellular Ca2+ mobilization (A) and 10 nM ionomycin-induced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
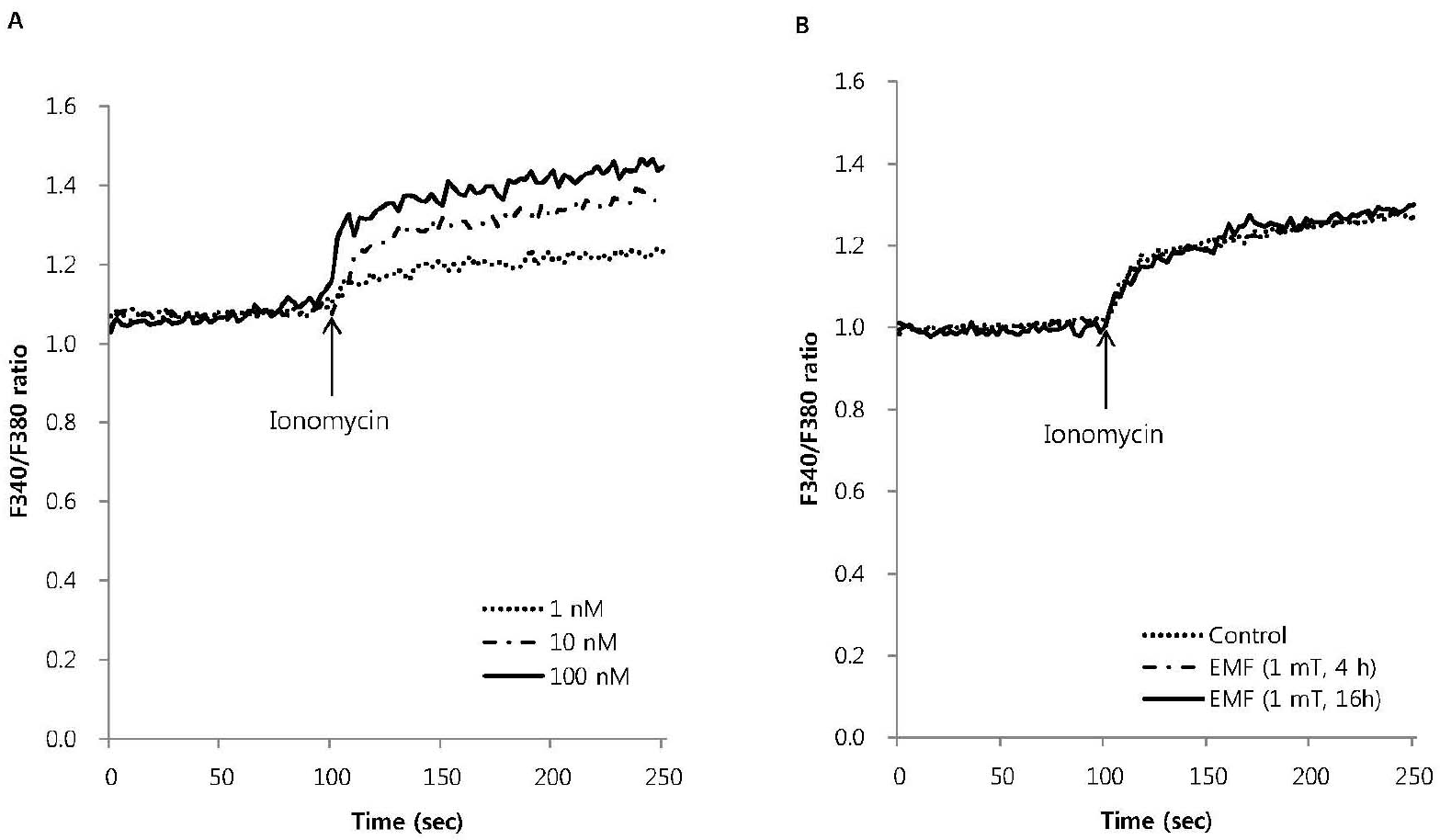
Fig. 4.
The effect of EMF on intracellular Ca2+ mobilization induced by thapsigargin in RBL 2H3 cells. Thapsigargin dose-dependently increased intracellular Ca2+ mobilization (A) and 100 nM thapsigargin-induced intracellular Ca2+ mobilization was not affected by EMF (60 Hz, 1 mT) for 4 or 16 h (B). Results are the representative data of four separate experiments.
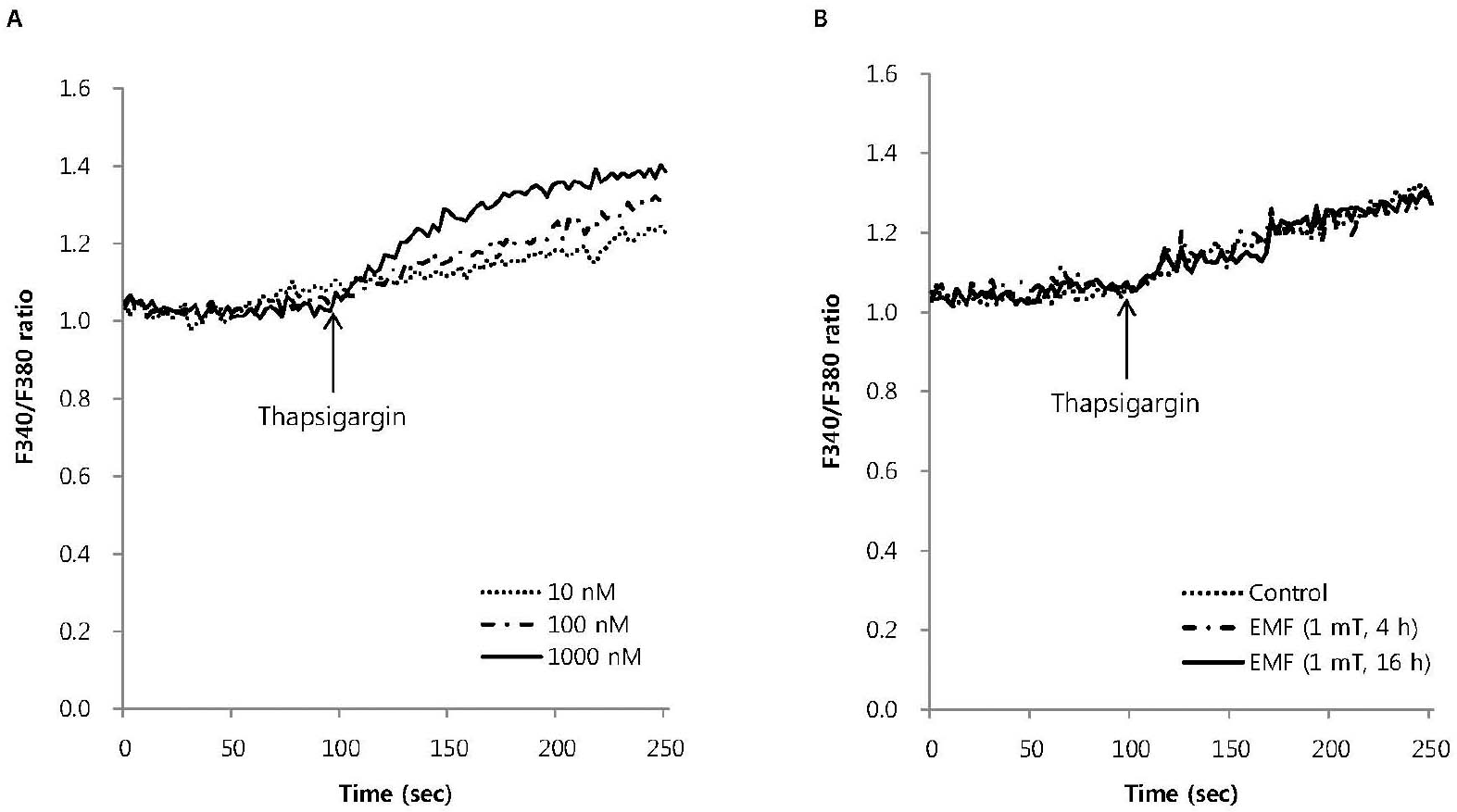
Fig. 5.
The effect of EMF on basal (A) and 1μM melittin-induced beta-hexosaminidase release (B) in RBL 2H3 cells. Both basal and 1μM melittin-induced beta-hexosaminidase release were not affected by exposure to EMF (60 Hz, 0.1 or 1 mT) for 4 or 16 h. Results indicate mean±S.D. from four separate experiments.
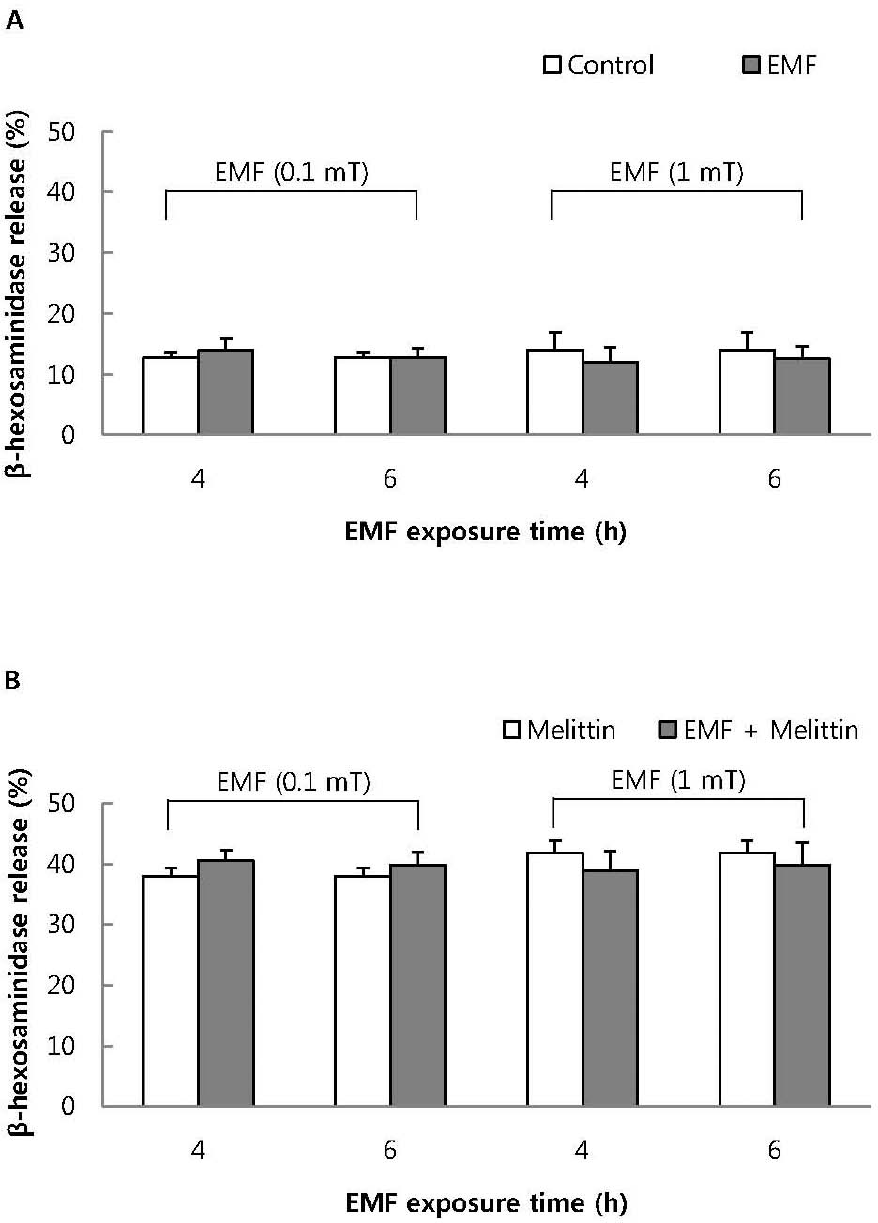
Table 1.
Dose-response of beta-hexosaminidase release by melittin, ionomycin and thapsigargin in RBL 2H3 cells
| Groups | Concentrations | % Release |
|---|---|---|
| Control | 14.8±1.6 | |
| Melittin | 0.1 μM | 18.3±1.2∗ |
| 0.5 μM | 28.8±4.1∗ | |
| 1.0 μM | 34.9±6.8∗ | |
| Ionomycin | 1 nM | 13.1±2.1 |
| 10 nM | 14.5±3.6 | |
| 100 nM | 15.2±1.7 | |
| Thapsigargin | 10 nM | 13.5±0.7 |
| 100 nM | 14.3±1.1 | |
| 1000 nM | 16.0±2.3 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download