Abstract
It was hypothesized that NaF induces calcium sensitization in Ca2+-controlled solution in permeabilized rat mesenteric arteries. Rat mesenteric arteries were permeabilized with β-escin and subjected to tension measurement. NaF potentiated the concentration-response curves to Ca2+ (decreased EC50 and increased Emax). Cumulative addition of NaF (4.0, 8.0 and 16 mM) also increased vascular tension in Ca2+-controlled solution at pCa 7.0 or pCa 6.5, but not at pCa 8.0. NaF-induced vasocontraction and GTPγS-induced vasocontraction were not additive. NaF-induced vasocontraction at pCa 7.0 was inhibited by pretreatment with Rho kinase inhibitors H1152 or Y27632 but not with a MLCK inhibitor ML-7 or a PKC inhibitor Ro31–8220. NaF induces calcium sensitization in a Ca2+-dependent manner in β-escin-permeabilized rat mesenteric arteries. These results suggest that NaF is an activator of the Rho kinase signaling pathway during vascular contraction.
References
1. Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007; 104:109–115.
2. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83:1325–1358.
3. Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rhokinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006; 27:97–104.

4. Murthy KS, Makhlouf GM. Fluoride activates G protein-dependent and -independent pathways in dispersed intestinal smooth muscle cells. Biochem Biophys Res Commun. 1994; 202:1681–1687.
5. Bogatcheva NV, Wang P, Birukova AA, Verin AD, Garcia JG. Mechanism of fluoride-induced MAP kinase activation in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L1139–1145.

6. Fujita A, Takeuchi T, Nakajima H, Nishio H, Hata F. Involvement of heterotrimeric GTP-binding protein and rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned muscle of guinea pig vas deferens. J Pharmacol Exp Ther. 1995; 274:555–561.
7. Yoshimura H, Jones KA, Perkins WJ, Kai T, Warner DO. Calcium sensitization produced by G protein activation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001; 281:L631–638.

8. Jeon SB, Jin F, Kim JI, Kim SH, Suk K, Chae SC, Jun JE, Park WH, Kim IK. A role for Rho kinase in vascular contraction evoked by sodium fluoride. Biochem Biophys Res Commun. 2006; 343:27–33.

9. Yang E, Jeon SB, Baek I, Chen ZA, Jin Z, Kim IK. 17beta-estradiol attenuates vascular contraction through inhibition of RhoA/Rho kinase pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009; 380:35–44.
10. Todoroki-Ikeda N, Mizukami Y, Mogami K, Kusuda T, Yamamoto K, Miyake T, Sato M, Suzuki S, Yamagata H, Hokazono Y, Kobayashi S. Sphingosylphosphorylcholine induces Ca2+-sensitization of vascular smooth muscle contraction: possible involvement of rho-kinase. FEBS Lett. 2000; 482:85–90.
11. Ryu SK, Ahn DS, Cho YE, Choi SK, Kim YH, Morgan KG, Lee YH. Augmented sphingosylphosphorylcholine-induced Ca2+-sensitization of mesenteric artery contraction in spontaneously hypertensive rat. Naunyn Schmiedebergs Arch Pharmacol. 2006; 373:30–36.
12. Choi SK, Ahn DS, Lee YH. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc Res. 2009; 82:324–332.

13. Durlu-Kandilci NT, Brading AF. Involvement of Rho kinase and protein kinase C in carbachol-induced calcium sensitization in beta-escin skinned rat and guinea-pig bladders. Br J Pharmacol. 2006; 148:376–384.
14. Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin-binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003; 369:117–128.
15. Chiba Y, Takeyama H, Sakai H, Misawa M. Effects of Y-27632 on acetylcholine-induced contraction of intact and permeabilized intrapulmonary bronchial smooth muscles in rats. Eur J Pharmacol. 2001; 427:77–82.

16. Johnson RP, El-Yazbi AF, Takeya K, Walsh EJ, Walsh MP, Cole WC. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009; 587:2537–2553.
17. Otto B, Steusloff A, Just I, Aktories K, Pfitzer G. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. J Physiol. 1996; 496:317–329.

18. Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins–ras-family or trimeric proteins or both–in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci U S A. 1996; 93:1340–1345.
19. Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005; 389:763–774.
20. Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in triton X-100-demembranated rabbit arterial smooth muscle. J Physiol. 1999; 520:139–152.
21. Kobayashi S, Somlyo AP, Somlyo AV. Guanine nucleotide- and inositol 1,4,5-trisphosphate-induced calcium release in rabbit main pulmonary artery. J Physiol. 1988; 403:601–619.

22. Antonny B, Sukumar M, Bigay J, Chabre M, Higashijima T. The mechanism of aluminum-independent G-protein activation by fluoride and magnesium. 31P NMR spectroscopy and fluorescence kinetic studies. J Biol Chem. 1993; 268:2393–2402.

23. Blackmore PF, Exton JH. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986; 261:11056–11063.

24. Cockcroft S, Taylor JA. Fluoroaluminates mimic guanosine 5′-[gammathio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987; 241:409–414.
25. Gilman AG. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J Clin Invest. 1984; 73:1–4.

26. Kanaho Y, Moss J, Vaughan M. Mechanism of inhibition of transducin GTPase activity by fluoride and aluminum. J Biol Chem. 1985; 260:11493–11497.

27. Bigay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985; 191:181–185.
28. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997; 389:990–994.

29. Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008; 14:64–68.

30. Lohn M, Plettenburg O, Ivashchenko Y, Kannt A, Hofmeister A, Kadereit D, Schaefer M, Linz W, Kohlmann M, Herbert JM, Janiak P, O'Connor SE, Ruetten H. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009; 54:676–683.
Fig. 1.
NaF induces Ca2+ sensitization in β-escin-permeabilized mesenteric arteries. Representative traces (A) show tension development when β-escin-permeabilized mesenteric arteries were exposed to increasing concentration of free calcium after initial high concentration of calcium (pCa 4.5) in the absence or presence of NaF (4.0, 8.0 or 12 mM). Line graphs (B) show the concentration-response curve to increasing concentration of free calcium (pCa 9.0∼ 5.5) in the absence or presence of NaF (4.0, 8.0 or 12 mM) in β-escinpermeabilized mesenteric arteries. Developed tension is expressed as a percentage of the maximum tension induced by pCa 4.5. Data are expressed as means of 5 experiments with vertical bars showing SEM. ∗∗p<0.01 vs. Ca2+ alone (Repeated measures ANOVA followed by post-hoc Dunnett test).
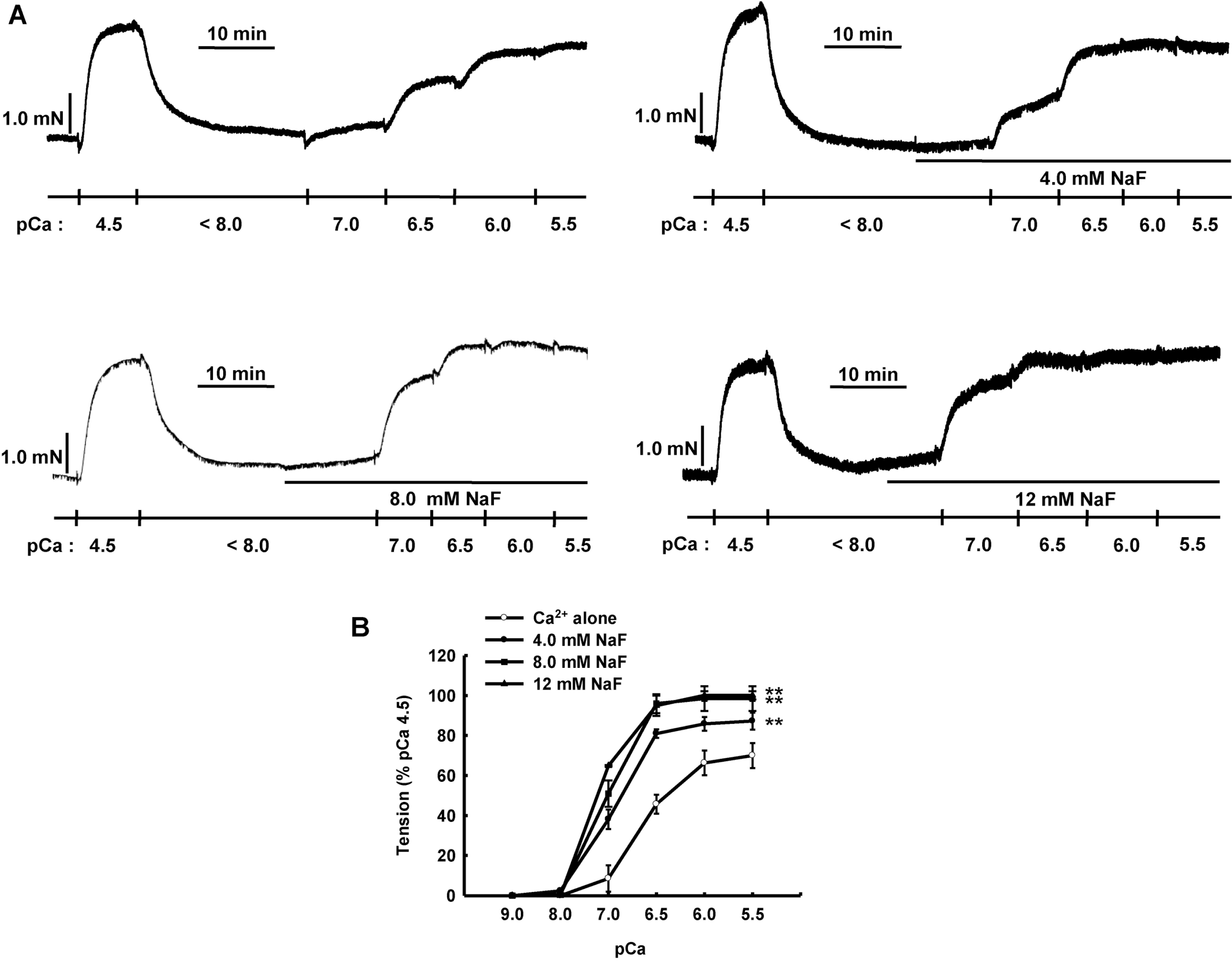
Fig. 2.
NaF induces vasocontraction in Ca2+-controlled solution in β-escin-permeabilized mesenteric arteries. Representative traces (A) show tension development when β-escin-permeabilized mesenteric arteries were exposed to cumulative addition of NaF (4.0, 8.0 and 16 mM) at constant concentration of calcium of pCa 8.0, 7.0 or 6.5. Bar graphs (B) show the developed tension elicited by cumulative addition of NaF (4.0, 8.0 and 16 mM) at constant concentration of calcium of pCa 8.0, 7.0 or 6.5 in β-escin-permeabilized mesenteric arteries. When NaF (16 mM)-induced contraction reached plateau, the bathing solutions were replaced with pCa 4.5 solution to obtain maximum contraction. Developed tension is expressed as a percentage of the maximum tension to pCa 4.5. Data are expressed as means of 5 experiments with vertical bars showing SEM.
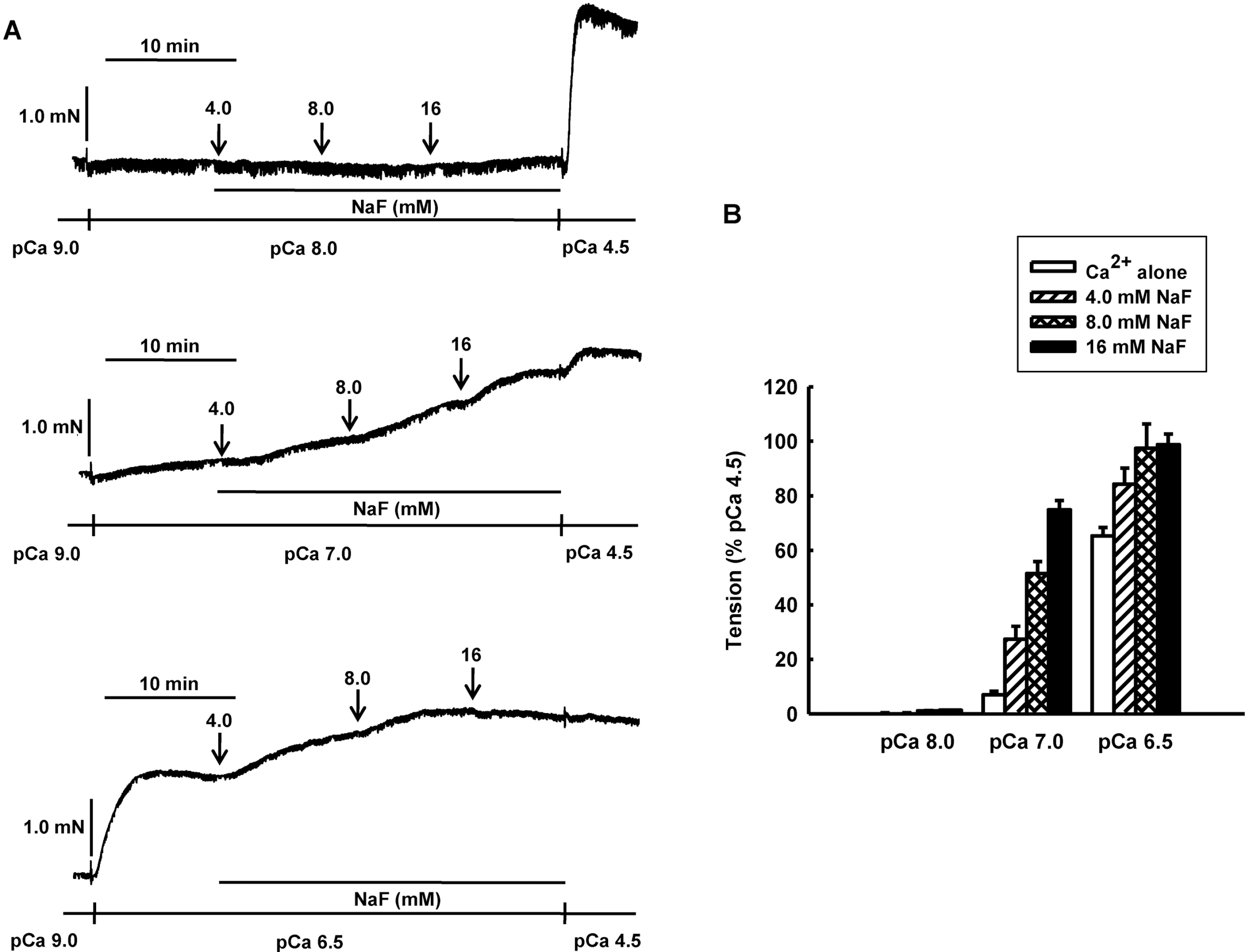
Fig. 3.
Effect of GTPγS on NaF-induced contraction in β-escinpermeabilized mesenteric arteries. Representative traces (A) show tension development when β-escinpermeabilized mesenteric arteries were exposed to addition of 100 μM GTP/S in the absence or presence of NaF (4.0 or 8.0 mM) at constant concentration of calcium of pCa 7.0. Bar graphs (B) show the developed tension elicited by addition of 100 μ M GTPγS in the absence or presence of NaF (4.0 or 12 mM) at constant concentration of calcium of pCa 7.0. When GTPγS (100 μM)-induced contraction reached plateau, the bathing solutions were replaced with pCa 4.5 solutions to obtain maximum contraction. Developed tension is expressed as a percentage of the maximum tension to pCa 4.5. Data are expressed as means of 5 experiments with vertical bars showing SEM. NS, not significant (p>0.05) (One-way ANOVA followed by post-hoc Dunnett test).
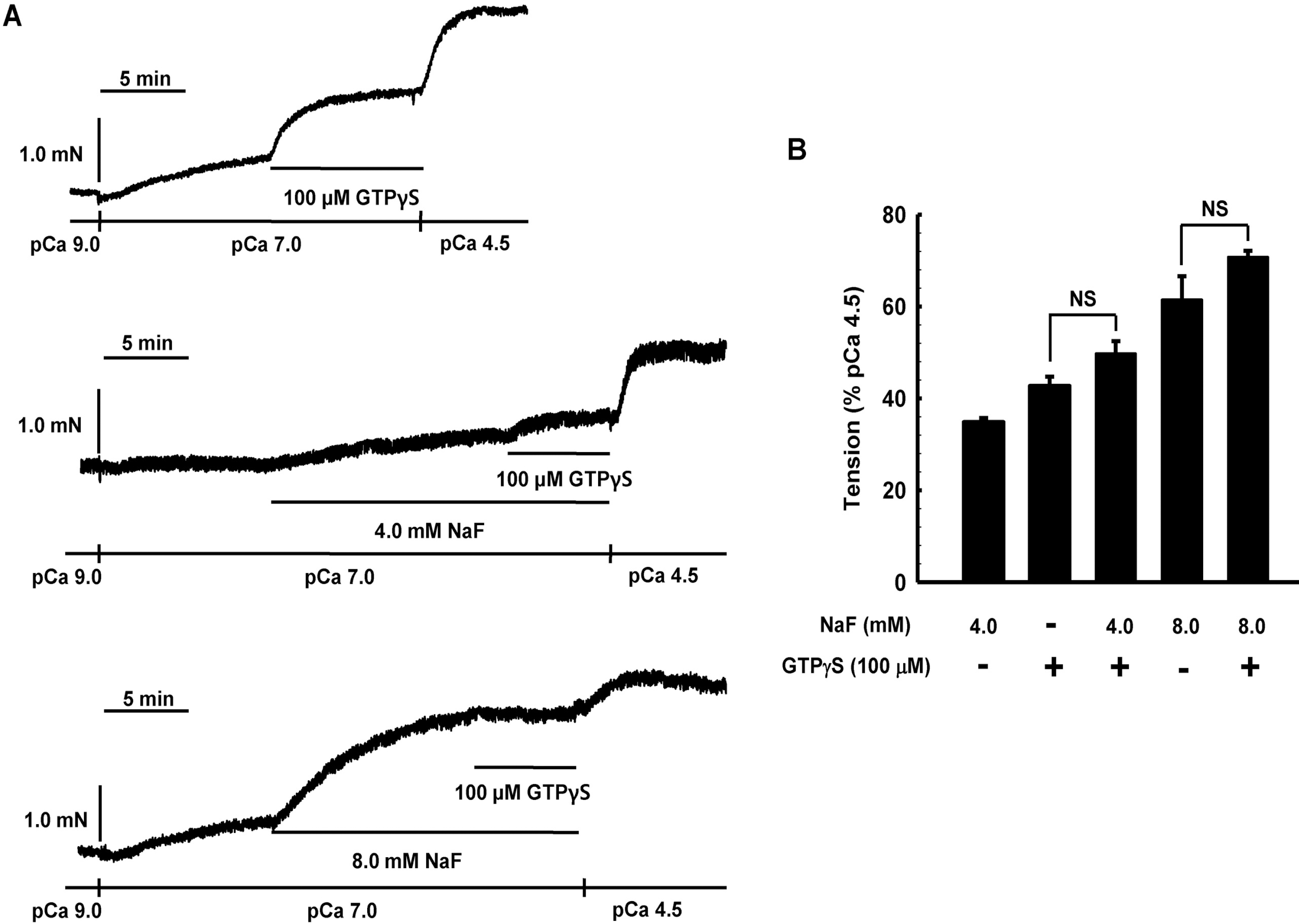
Fig. 4.
Effect of GDPβS on NaF-induced contraction in β-escin-perme-abilized mesenteric arteries. Representative traces (A) show tension development when β-escin-permeabilized mesenteric arteries were exposed to addition of 8.0 mM NaF or 100 μM GTPγS following 300 μM GDPβS at constant concentration of calcium of pCa 7.0. Representative traces (B) show tension development when β-escin-permeabilized mesenteric arteries were exposed to addition of 8.0 mM NaF or 100 μM GTPγS in the absence or presence of 300 μM GDPβS at constant concentration of calcium of pCa 7.0. When GTPγS (100 μM)- or NaF (8.0 mM)-induced contraction reached plateau, the bathing solutions were replaced with pCa 4.5 solutions to obtain maximum contraction. The traces are representative of four experiments.
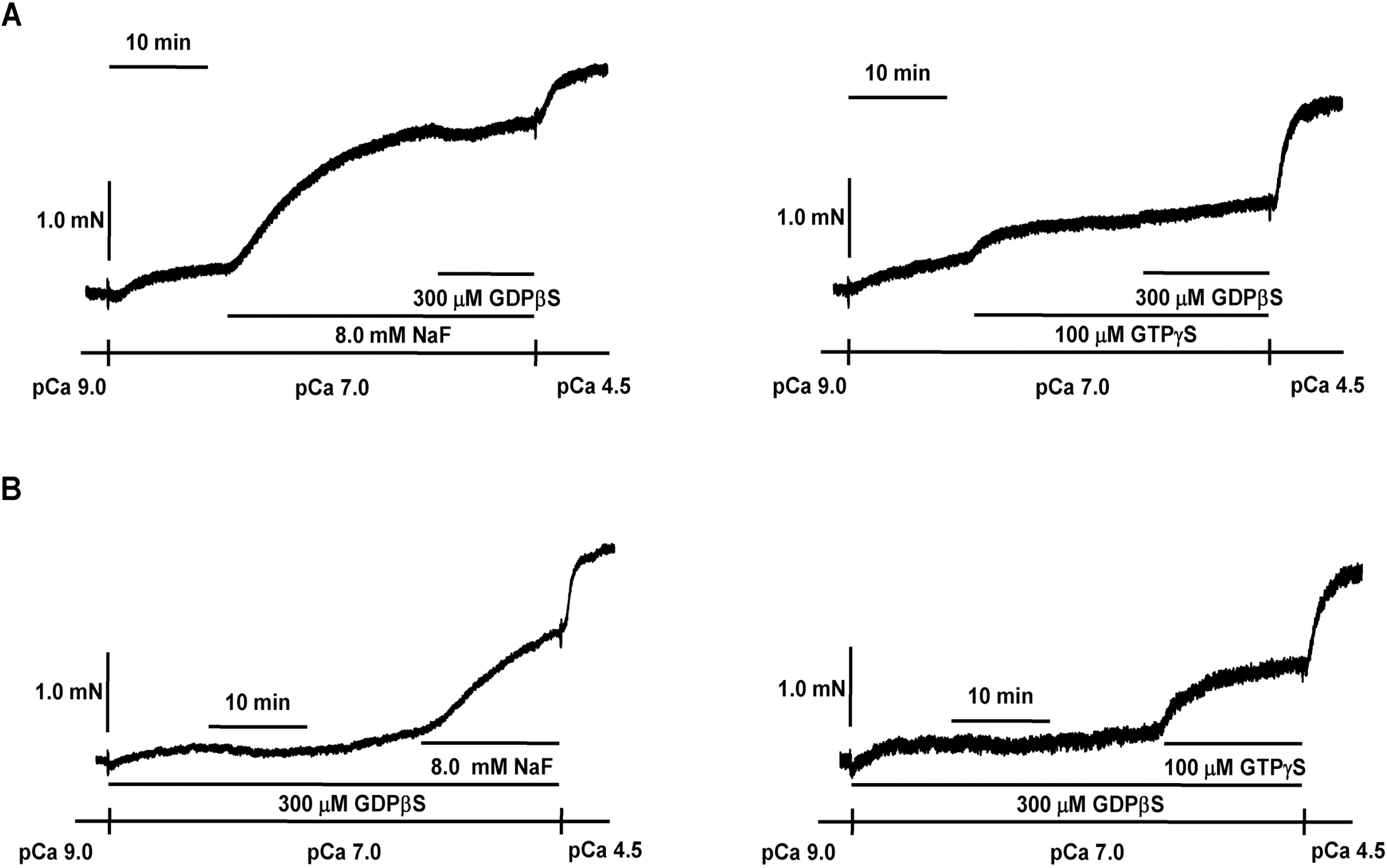
Fig. 5.
Effect of Rho kinase inhibitors, Y27632 and H1152 on NaF-induced vasocontraction in β-escin-perme-abilized mesenteric arteries. Representative traces (A) show tension development when β-escin-perme-abilized mesenteric arteries were exposed to addition of 8.0 mM NaF in the absence or presence of Rho kinase inhibitors, Y27632 or H1152, at constant concentration of calcium of pCa 7.0. Bar graphs (B) show the developed tension elicited by addition of 8.0 mM NaF in the absence or presence of Rho kinase inhibitors, Y27632 or H1152, at constant concentration of calcium of pCa 7.0 in β-escin-permeabilized mesenteric arteries. When NaF (8.0 mM)-induced contraction reached plateau, the bathing solutions were replaced with pCa 4.5 solution to obtain maximum contraction. Developed tension is expressed as a percentage of the maximum tension to pCa 4.5. Data are expressed as means of 5 experiments with vertical bars showing SEM. ∗∗p<0.01 vs. vehicle (One-way ANOVA followed by post-hoc Dunnett test).
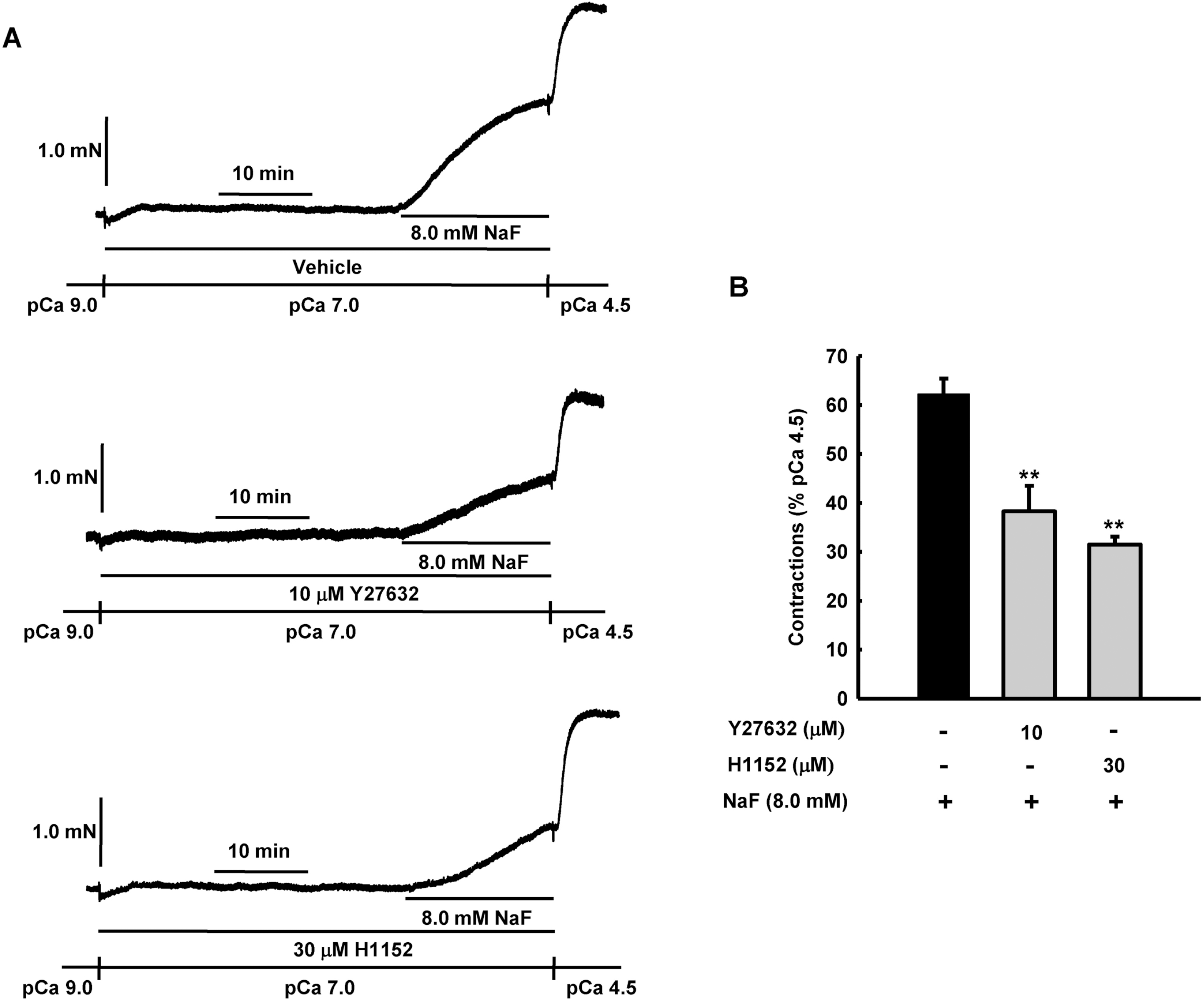
Fig. 6.
Effect of ML-7 or Ro31–8220 on NaF-induced vasocontraction in β-escin-permeabilized mesenteric arteries. Representative traces (A) show tension development when β-escin-permeabilized mesenteric arteries were exposed to addition of 8.0 mM NaF in the absence or presence of MLCK inhibitor ML-7 or PKC inhibitor Ro31-8220 at constant concentration of calcium of pCa 7.0. Bar graphs (B) show the developed tension elicited by addition of 8.0 mM NaF in the absence or presence of MLCK inhibitor ML-7 or PKC inhibitor Ro31–8220 at constant concentration of calcium of pCa 7.0 in β-escin-permeabilized mesenteric arteries. When NaF (8.0 mM)-induced contraction reached plateau, the bathing solutions were replaced with pCa 4.5 solution to obtain maximum contraction. Developed tension is expressed as a percentage of the maximum tension to pCa 4.5. Data are expressed as means of 5 experiments with vertical bars showing SEM. NS, not significant (p>0.05) (One-way ANOVA followed by post-hoc Dunnett test).
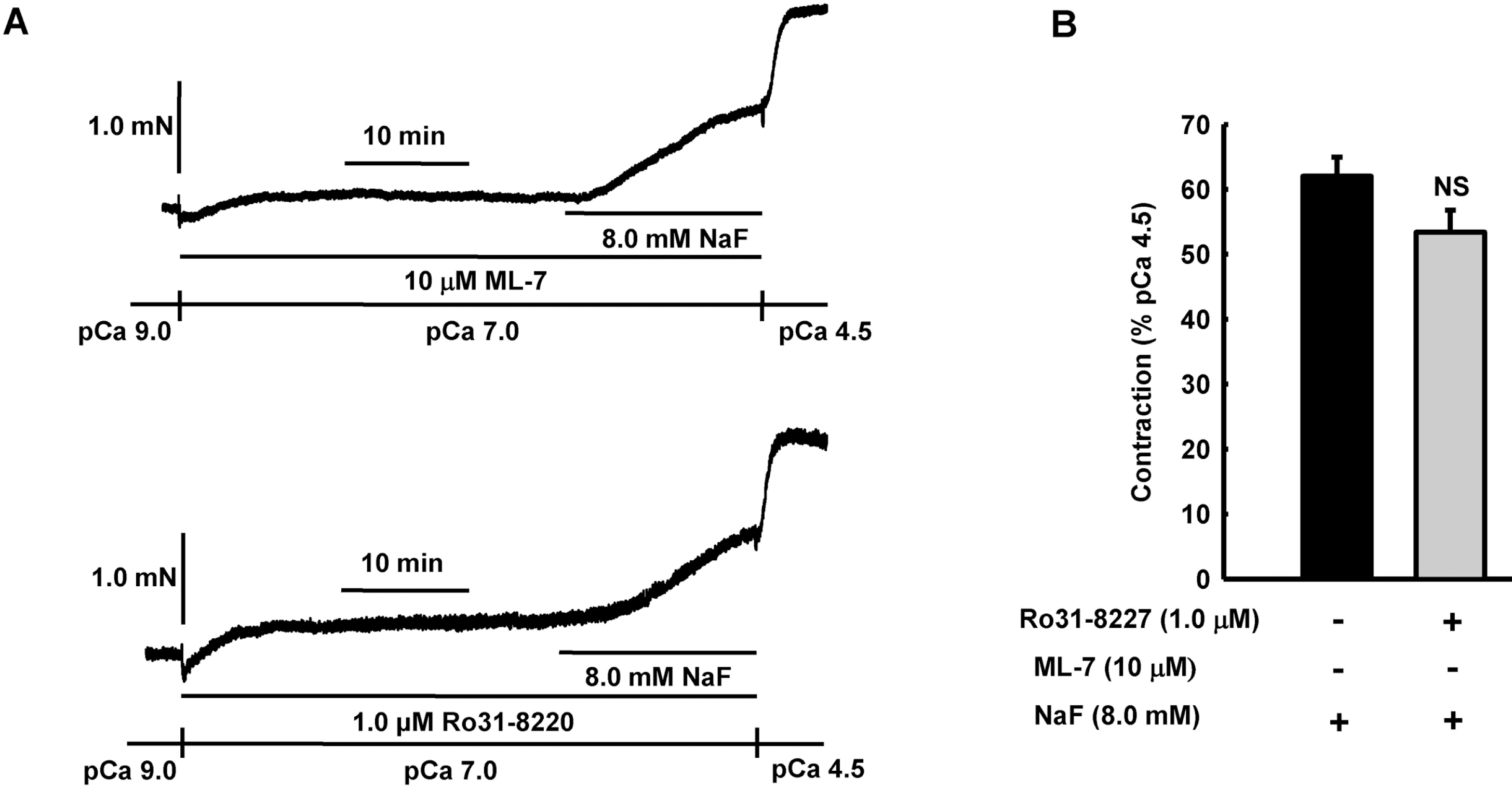
Table 1.
EC50 and maximal effect (Emax) of the concentration-response curves to Ca2+ in the absence or presence of NaF in β-escin-permeabilized rat mesenteric arteries
| EC50 (pCa) | Emax (% of pCa 4.5) | |
|---|---|---|
| Ca2+ alone | 6.6±0.03 | 69±4.5 |
| 4.0 mM NaF | 7.0±0.01∗ | 87±4.0∗ |
| 8.0 mM NaF | 7.0±0.01∗ | 98±6.2∗ |
| 12 mM NaF | 7.1±0.06∗ | 100±2.6∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download