Abstract
The binding of interleukin-6 (IL-6) cytokine family ligands to the gp130 receptor complex activates the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) signal transduction pathway, where STAT3 plays an important role in cell survival and tumorigenesis. Constitutive activation of STAT3 has been frequently observed in many cancer tissues, and thus, blocking of the gp130 signaling pathway, at the JAK level, might be a useful therapeutic approach for the suppression of STAT3 activity, as anticancer therapy. AG490 is a tyrphostin tyrosine kinase inhibitor that has been extensively used for inhibiting JAK2 in vitro and in vivo. In this study, we demonstrate a novel mechanism associated with AG490 that inhibits the JAK/STAT3 pathway. AG490 induced downregulation of gp130, a common receptor for the IL-6 cytokine family compounds, but not JAK2 or STAT3, within three hours of exposure. The downregulation of gp130 was not caused by enhanced degradation of gp130 or by inhibition of mRNA transcription. It most likely occurred by translation inhibition of gp130 in association with phosphorylation of the eukaryotic initiation factor-2α. The inhibition of protein synthesis of gp130 by AG490 led to immediate loss of mature gp130 in cell membranes, due to its short half-life, thereby resulting in reduction in the STAT3 response to IL-6. Taken together, these results suggest that AG490 blocks the STAT3 activation pathway via a novel pathway.
REFERENCES
Aggarwal BB., Sethi G., Ahn KS., Sandur SK., Pandey MK., Kunnumakkara AB., Sung B., Ichikawa H. Targeting signal- transducer-and-activator-of-transcription (STAT)-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 1091:151–169. 2006.
Bertolotti A., Zhang Y., Hendershot LM., Harding HP., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2:326–332. 2000.

Ferrajoli A., Faderl S., Van Q., Koch P., Harris D., Liu Z., Hazan-Halevy I., Wang Y., Kantarjian HM., Priebe W., Estrov Z. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 67:11291–11299. 2009.

Gerhartz C., Dittrich E., Stoyan T., Rose-John S., Yasukawa K., Heinrich PC., Graeve L. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem. 223:265–274. 1994.

Germain D., Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 13:5665–5669. 2007.
Graf D., Haselow K., Münks I., Bode JG., Häussinger D. Caspase-mediated cleavage of the signal-transducing IL-6 receptor subunit gp130. Arch Biochem Biophys. 477:330–338. 2008.

Graf D., Kohlmann C., Haselow K., Gehrmann T., Bode JG., Häussinger D. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and -independent pathways in rat liver. Hepatology. 44:1206–1217. 2006.

Harding HP., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.

Heinrich PC., Behrmann I., Haan S., Hermanns HM., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 374:1–20. 2003.

Hideshima T., Chauhan D., Hayashi T., Akiyama M., Mitsiades N., Mitsiades C., Podar K., Munshi NC., Richardson PG., Anderson KC. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 22:8386–8393. 2003.

Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 16:79–86. 2001.

Lee HK., Seo IA., Suh DJ., Lee HJ., Park HT. A novel mechanism of methylglyoxal cytotoxicity in neuroglial cells. J Neurochem. 108:273–284. 2009a.

Lee HK., Seo IA., Shin YK., Park JW., Suh DJ., Park HT. Capsaicin inhibits the IL-6/STAT3 pathway by depleting intracellular gp130 pools through endoplasmic reticulum stress. Biochem Biophys Res Commun. 382:445–450. 2009c.

Lee HK., Seo IA., Suh DJ., Hong JI., Yoo YH., Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 108:776–786. 2009b.

Meydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder JS., Freedman M., Cohen A., Gazit A., Levitzki A., Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 379:645–648. 1996.

Miyamoto N., Sugita K., Goi K., Inukai T., Lijima K., Tezuka T., Kojika S., Nakamura M., Kagami K., Nakazawa S. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia. 15:1758–1768. 2001.

Opdam FJ., Kamp M., de Bruijn R., Roos E. Jak kinase activity is required for lymphoma invasion and metastasis. Oncogene. 23:6647–6653. 2004.

Rahaman SO., Harbor PC., Chernova O., Barnett GH., Vogelbaum MA., Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 55:8404–8413. 2002.

Samanta AK., Lin H., Sun T., Kantarjian H., Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 66:6468–6472. 2006.

Satriotomo I., Bowen KK., Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 98:1353–1368. 2006.

Scholz A., Heinze S., Detjen KM., Peters M., Welzel M., Hauff P., Schirner M., Wiedenmann B., Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 125:891–905. 2003.

Shyu WC., Lin SZ., Chiang MF., Chen DC., Su CY., Wang HJ., Liu RS., Tsai CH., Li H. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest. 118:133–148. 2008.

Szegezdi E., Logue SE., Gorman AM., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880–885. 2006.

Tanaka Y., Tanaka N., Saeki Y., Tanaka K., Murakami M., Hirano T., Ishii N., Sugamura K. c-Cbl-dependent monoubiquitination and lysosomal degradation of gp130. Mol Cell Biol. 28:4805–4818. 2008.

Tebbutt NC., Giraud AS., Inglese M., Jenkins B., Waring P., Clay FJ., Malki S., Alderman BM., Grail D., Hollande F., Heath JK., Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 8:1089–1097. 2002.

Fig. 1.
AG490 inhibited IL-6-induced STAT3 activation. (A) AG490 (50 μM) suppressed tyrosine phosphorylation of STAT3 by IL-6 in a time-dependent manner without affecting STAT3 protein levels. AG490 or AG1478 (50 μM) was pretreated for the indicated times, and then stimulated with IL-6 for 15 min. (B) Quantitative results showing the change in pSTAT3 levels following AG490 treatment for the indicated times. (C) Luciferase promoter assay. RT4 cells were transfected with a GFAP promoter-luciferase reporter construct (GF1L). One day after transfection, the cells were pretreated with AG490 (50 μM) for 2 hrs, and then simulated with IL-6 for 6 hrs. The results are expressed as a relative value to the untreated controls. Data are the mean±S.E. from three independent experiments. (D) Temporal profile of tyrosine phosphorylation of STAT3 and JAK2 by IL-6. The cells were treated with IL-6 (50 ng/ml) for the indicated times, and then Western blot analysis was performed to examine tyrosine phosphorylation of STAT3 and JAK2. b-tub; beta-tubulin. ∗p<0.05.
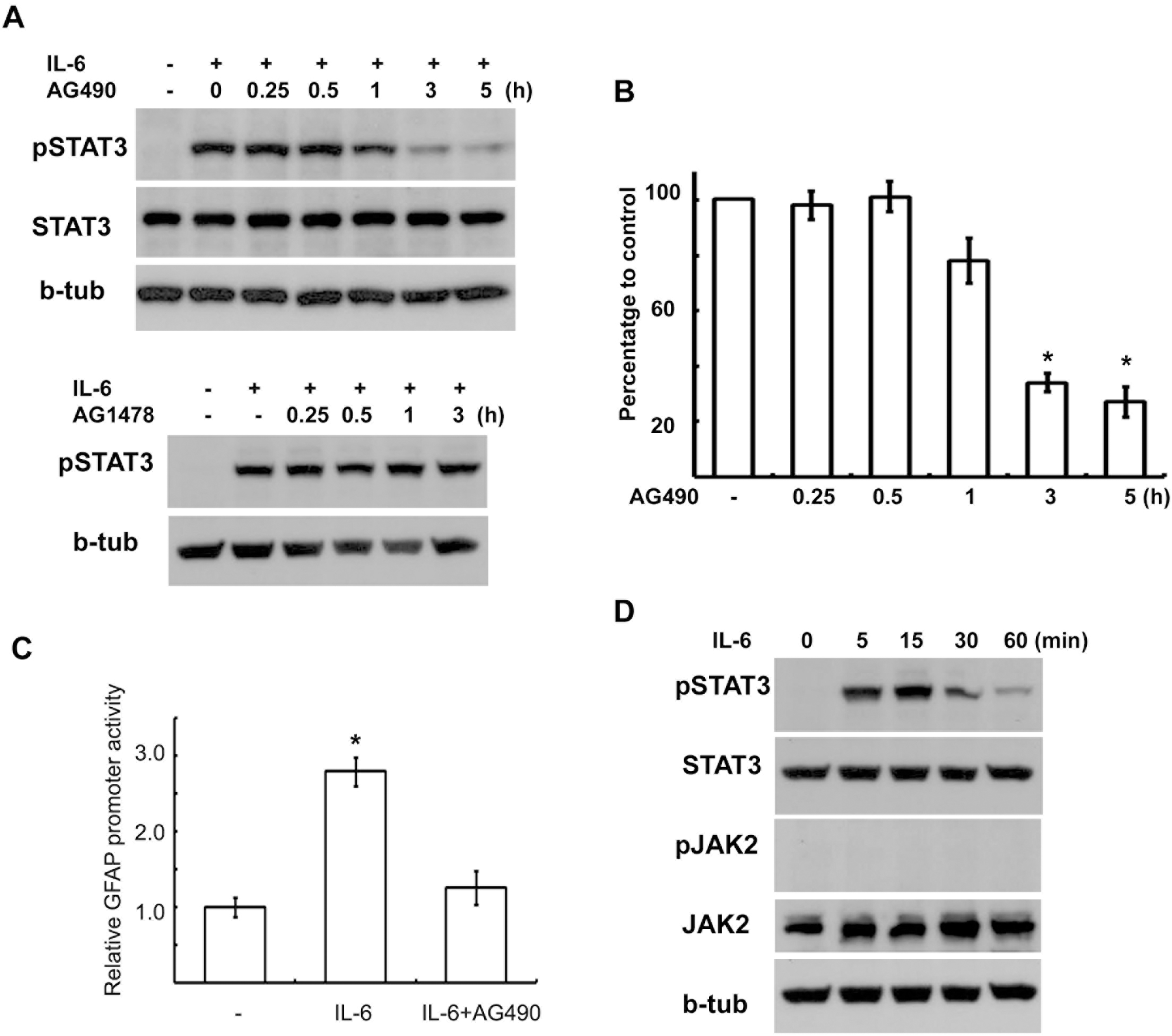
Fig. 2.
AG490 downregulates gp130 expression. (A, B) RT4 cells were treated with AG490 (50 μM) or AG1478 (50 μM) for the indicated times, and then Western blot analysis was performed to examine the protein levels of gp130, JAK2, Unc5b and beta-tubulin. (B) Quantitative results showing the changes in gp130 levels following AG490 or AG1478 treatment. gp130 showed two bands with different molecular weights, and the intensity values of each of two gp130 bands were added for analysis. (C) A dose-dependent downregulation of gp130 by AG490 treatment. (D) RT4 cells were treated with AG490 (50 μM) for the indicated time, and total RNA was extracted for RT-PCR analysis. GAPDH mRNA levels were used as a control. Quantitative data represent the means±S.E of three independent experiments. ∗p<0.05.
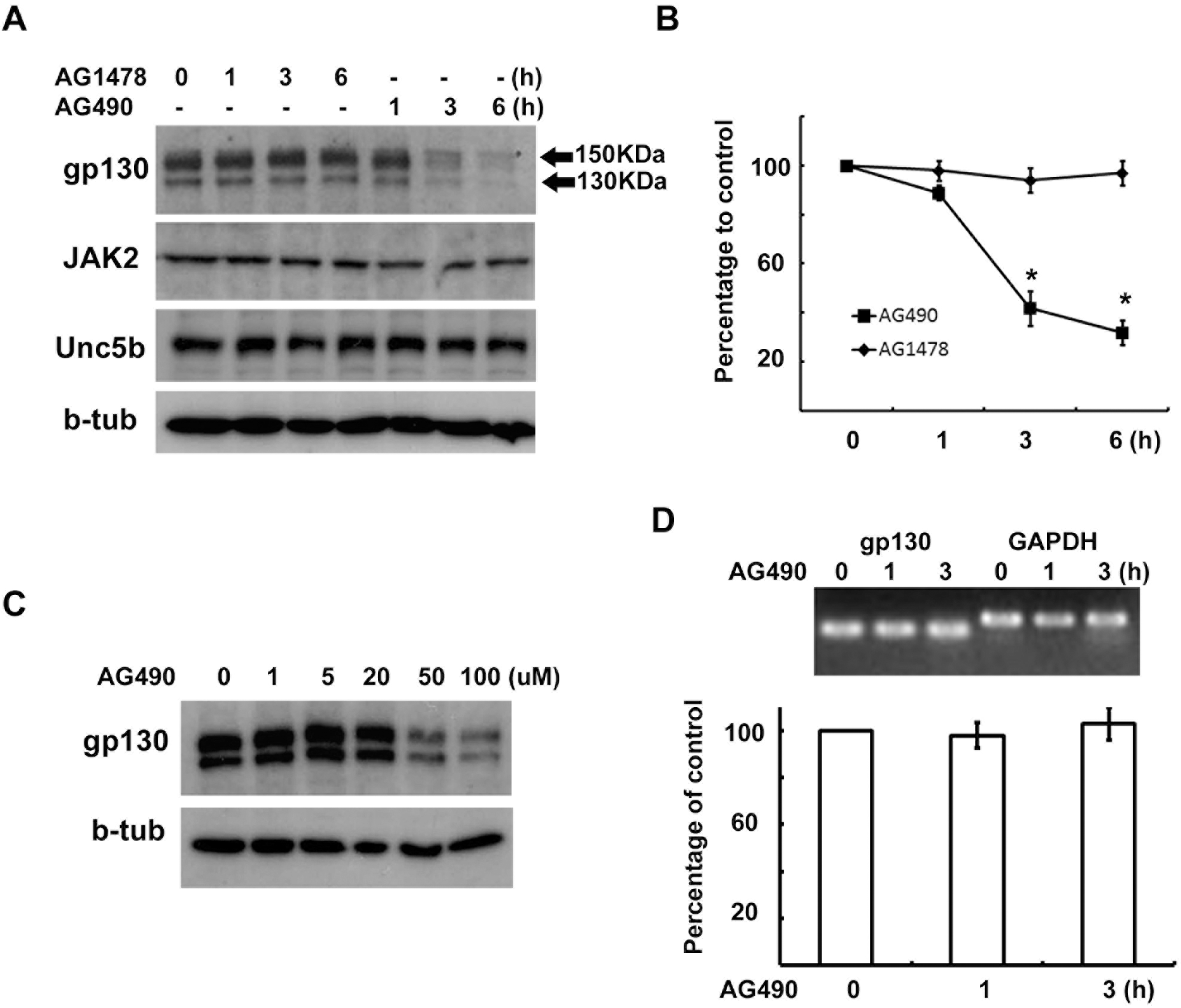
Fig. 3.
Degradation of gp130 was not enhanced by AG490. (A) Cell surface proteins were biotinylated at 4°C, and then cells were returned to 37°C for the indicated time in the presence or absence of AG490 (50 μM). Protein extracts were precipitated with streptavidin-agarose beads, and analyzed by Western blotting with an antibody against gp130. (B) Quantitative results showing the change in biotinylated gp130 levels following AG490 treatment. (C) Cells were pre-treated with protease inhibitors for 30 min and then cells were exposed to AG490 (50 μM) for 3 hrs. (D) Quantitative results showing the change in gp130 levels following AG490 treatment in the presence of several protease inhibitors. Leup; leupeptin, MG; MG132, NH4; NH4Cl, CQ; chloroquine. Data represent the means± S.E of three independent experiments. ∗p<0.05.
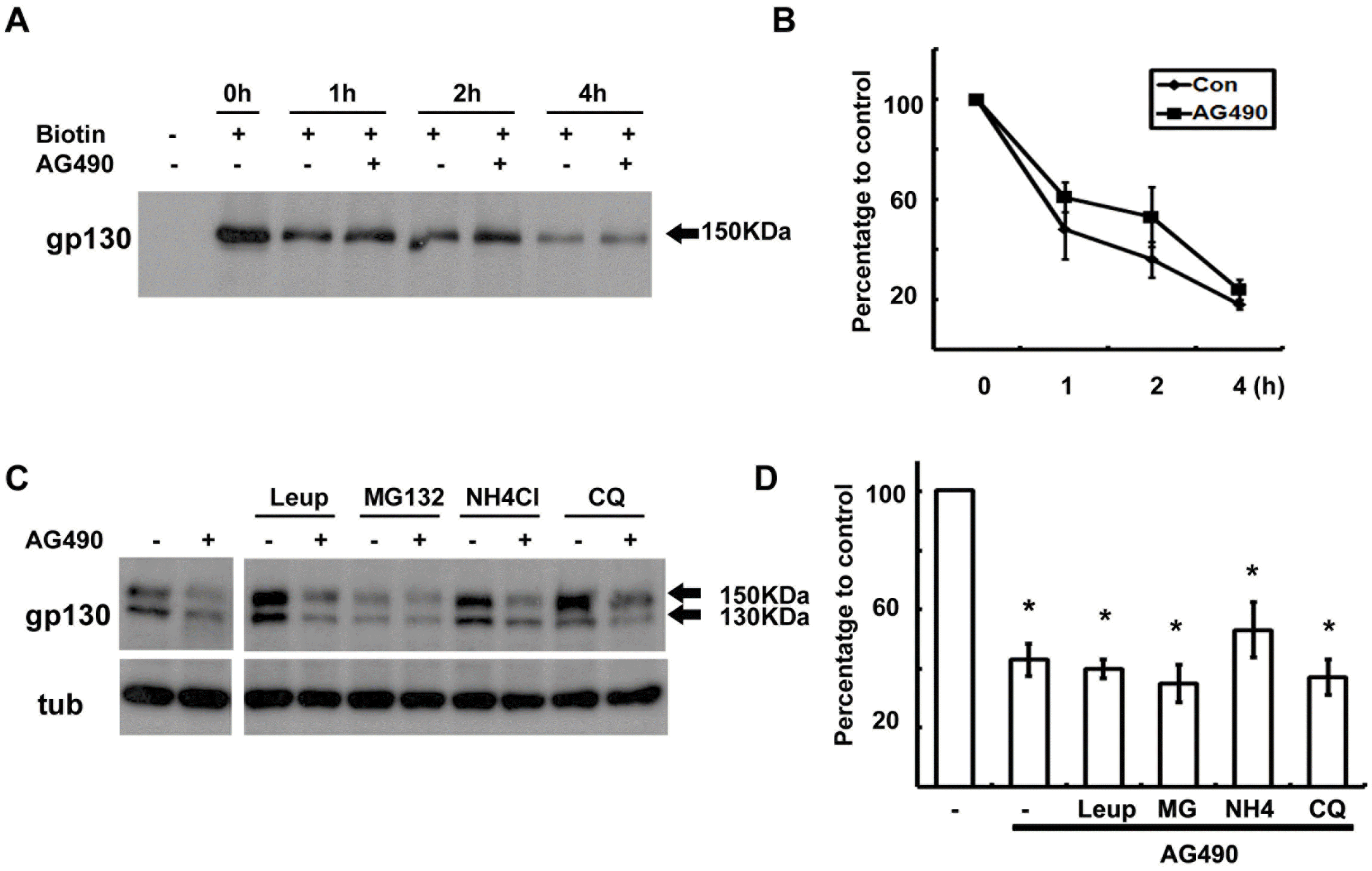
Fig. 4.
Protein synthesis of gp130 is suppressed by AG490. (A) Cells were pretreated with cycloheximide (CHX, 10 μM) for 4 hrs, and then washed and reincubated in normal growth medium with or without AG490 (50 μM) for the indicated times. W1; reincubation in normal medium for 1 hr after 4 hrs of CHX treatment. (B) Quantitative results showing the change in gp130 levels following CHX treatment.
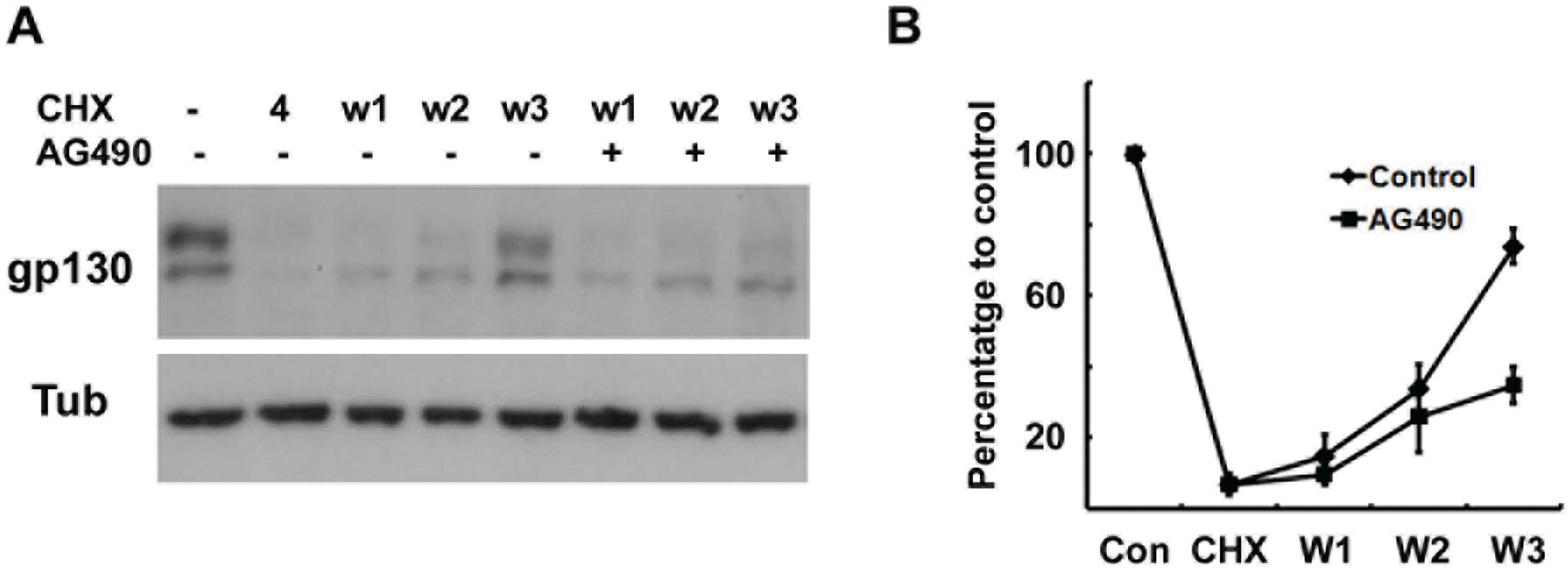
Fig. 5.
Phosphorylation of eIF-2α by AG490. (A) Temporal profile of phosphorylation of eIF-2α and induction of GADD153 (GADD) by AG490 (50 μM). The cells were treated with AG490 for the indicated times, and then Western blot analysis was performed. (B) Cells were treated with an ER stress inducer dithiothreitol (DTT, 3 mM) for the indicated times, and protein levels of gp130, peIF-2α and GADD153 were analyzed by Western blotting.
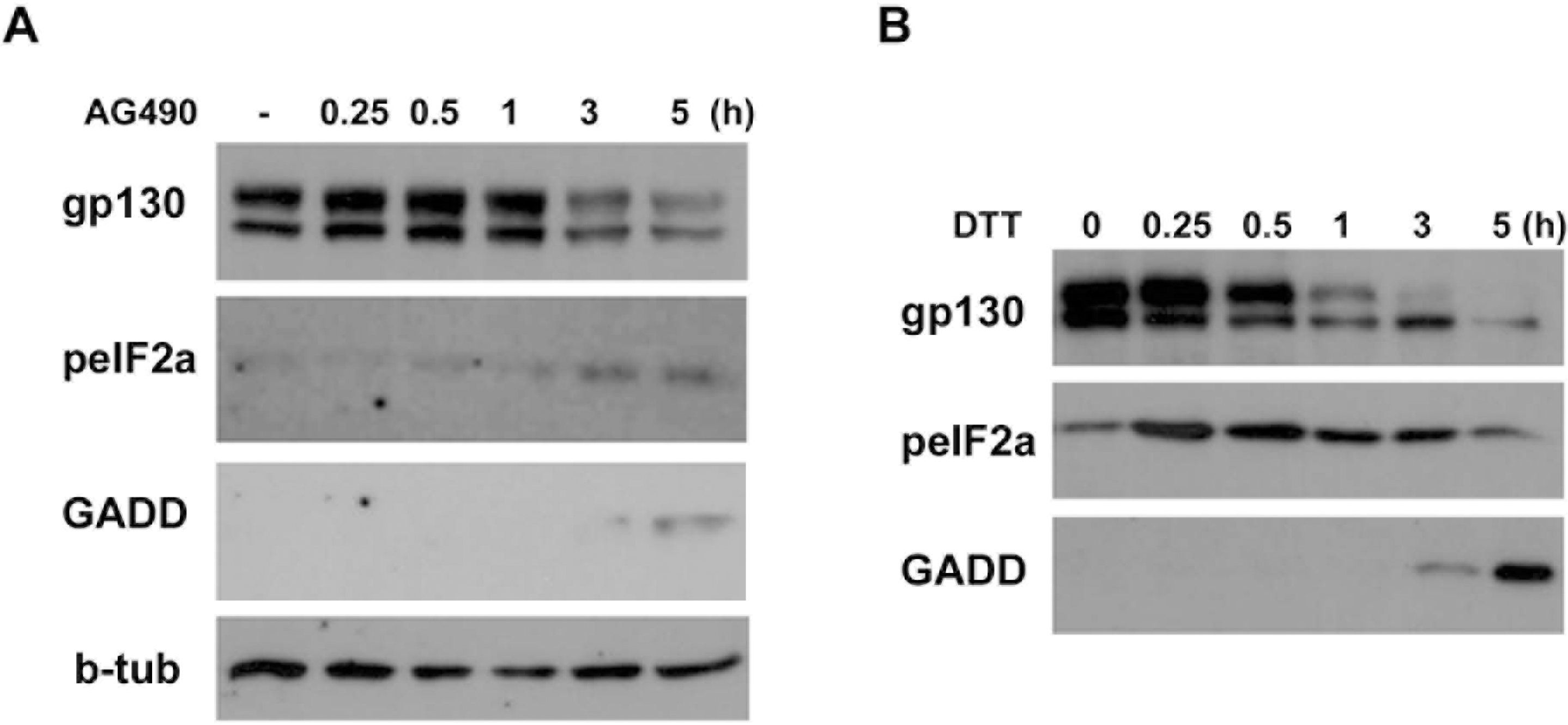
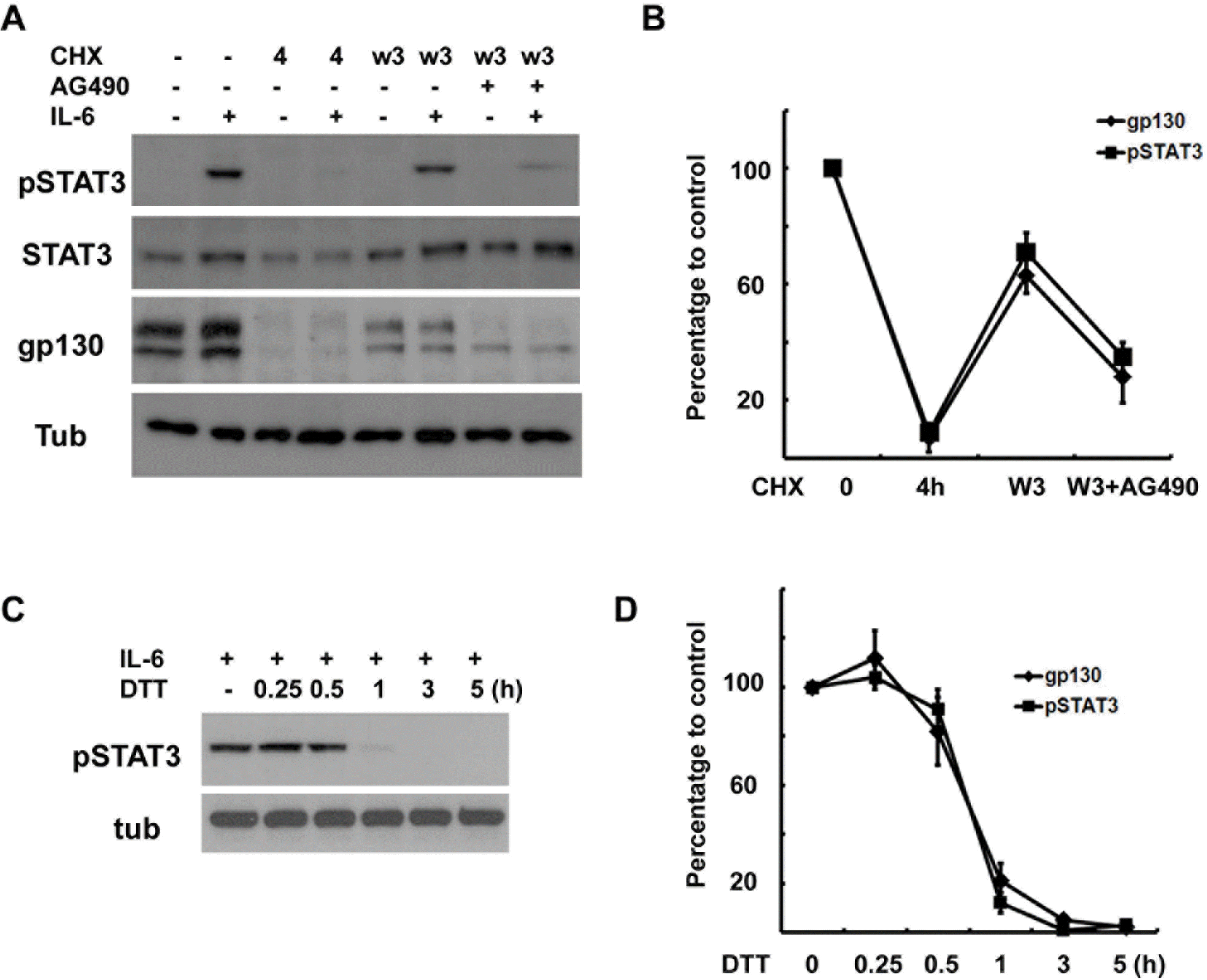
Fig. 6.
Correlation between gp130 levels and IL-6 response. (A) Cells were pretreated with CHX (10 μM) for 4 hrs, and then washed and rein-cubated in normal growth medium with or without AG490 (50 μM) for 3 hrs, and then IL-6 (50 ng/ml) was added for 15 min. The protein extracts were analyzed by Western blotting. (B) Quantitative results showing the changes in gp130 and pSTAT3 levels. Data represent the means±S.E of three independent experiments. (C) The cells were pretreated with dithiothreitol (DTT, 3 mM) for the indicated times and then stimulated with IL-6 for 15 min. Protein extracts were analyzed by Western blotting for pSTAT3. (D) Quantitative results showing the changes in gp130 and pSTAT3 levels in a time-dependent manner. Data represent the means±S.E of three independent experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download