Abstract
Purpose
This study was intended to investigate the migrating motor complex (MMC) changes after ileal bypass in ex-vivo mouse models.
Methods
Partial (side-to-side) and total bypass (occlusion of proximal part of bypassed loop) were performed on ileums of female Institute of Cancer Research mice. After 2 and 4 weeks, the bypassed segments were harvested and MMCs were recorded at 4 different sites ex-vivo. Amplitude, duration, interval, direction of propagation, and the area under the curve (AUC) of MMCs were measured and compared to those of the controls.
Results
In control mice (n = 7), most MMCs propagated aborally (91.1%). After 2 weeks of partial bypass (n = 4), there was a significant decrease in both amplitude and AUC, and orally-propagating MMCs increased significantly (45%, P = 0.002). Bidirectional MMCs (originating in the bypassed loop and propagating in both directions) were also observed (10%). The amplitude of the MMCs remained decreased at 4 weeks after partial bypass (n = 4), and neither the AUC nor the direction of propagation showed significant changes compared to 2 weeks. Similarly, in the total bypass model, both the amplitude and AUC of the MMCs decreased significantly compared to controls. In contrast to partial bypass, 95% of the MMCs within the bypassed loop propagated aborally after 2 weeks (n = 6), which was similar to the control state. After 4 weeks (n = 5), however, MMCs either lost their temporal relationship or completely disappeared.
In surgical practice, small to small or small to large bowel bypass is performed for various settings such as radiation enteritis or Crohn's disease. However, blind loop syndrome is a well-documented complication following the side-to-side anastomosis of the intestine. Stagnation of the intestinal contents and subsequent bacterial overgrowth in the bypassed loop has been known to be the cause [1]. Re-entry of the intestinal contents through the lateral anastomosis into the bypass loop might be related to the stagnation [2]. Clinically, some surgeons used proximal loop ligation during the loop esophago-jejunostomy to prevent this phenomenon with some success [3]. However, no clear mechanism for development of blind loop syndrome or beneficial role of proximal loop ligation during the intestinal bypass procedure has been demonstrated.
Migrating motor complex (MMC) describes organized groups of contractions periodically migrating down both the small and large intestine, and has been observed in a variety of animals, including humans [4,5]. MMC is one of the key elements in gastrointestinal motility, and has been known to act as an intestinal house-keeper; periodically cleansing the bowel in preparation for the next meal. Ever since Wood [6] first described the MMC in the isolated mouse colon and Bush et al. [7] described the MMC in the isolated mouse ileum, MMC has been most intensively studied in the mouse model ex-vivo.
We hypothesized that the mechanism underlying the stagnation of the intestinal contents in the blind loop syndrome might be related to changes in MMC, and so we measured the MMCs after creating a side-to-side ileal bypass anastomosis in a mouse and extrapolated the mechanism of the stagnation through the ex-vivo experiments.
Female Institute of Cancer Research mice, aged 8 weeks, were used for the ileal side-to-side anastomosis. The animals were purchased from specific pathogen free laboratory animal company (Koatech, Pyeongtaek, Korea). We did not restrict diet and no bowel preparation was performed pre-operatively. The mice were anesthetized with a single i.p. dose of tiletamine/zolazepam (Zoletil, Virbac Laboratories, Carros, France) 40 mg/kg. Single s.c. dose of atropine (Daihan Pharm Co., Seoul, Korea) 0.04 mg/kg was administered to decrease the tracheal secretion to prevent asphyxia and single i.p. dose of cefmetazole (Daewoong Co., Seoul, Korea) 50 mg/kg was administered. When the absence of a hindlimb pinch-withdrawal reflex was verified, a median laparotomy was performed. After identifying the appendix and ileo-cecal junction, the ileum was traced proximally to locate 4 cm and 8 cm from ileo-cecal junction. After opening the antemesenteric border about 3 mm, side-to-side anastomosis was performed using 10-0 monofilament suture (Ethilon, Ethicon Inc., Sommerville, NJ, USA) under magnifying (×5) surgical loupe. The anterior and the posterior rows were sutured in an interrupted manner for five stitches, respectively (partial bypass model; Fig. 1A). For a total bypass model, the proximal portion of the bypass loop was doubly ligated to create obstruction with the same suture material, without compromising the mesenteric vascular arcade (Fig. 1B). After completion of the anastomosis, the intestine was replaced within the abdomen and the abdomen was closed with 5-0 silk suture. The animals were allowed to recover post-operatively on a heated blanket. Throughout the surgery and recovery the animals were intermittently oxygenated with a 97% O2-3% CO2 mixture. Operated animals were sacrificed by CO2 inhalation followed by cervical dislocation, for experiments 2 weeks or 4 weeks after the operation. All the procedures performed on the animals were approved by the Institute of Laboratory Animal Resources (No. SNU-080415-1).
After the animals were sacrificed, the bypass loop was removed, including the 2 cm segment of intestine proximal and distal to the anastomosis, and placed in Krebs-Ringer buffer (KRB). The luminal contents were flushed gently with KRB and the mesenteric tissues were cleared by sharp dissection, avoiding the injuries to the intestinal wall. We designed a loop-holding frame with two S-shaped stainless rods and the intestine was held securely within this frame fixed into the bath chamber. The bath chamber was continuously perfused with warmed (37.0 ± 0.5℃) KRB, flowing at a rate of 10 mL/min and gassed (97% O2-3% CO2) throughout the experiment. For the control mice, ileum of the same portion (8 cm segment) were removed and fixed into the bath using straight stainless rod (1.5 mm diameter).
Four stainless steel clips (4 mm, micro-serrefines; Fine Science tools Inc., Foster City, CA, USA) were attached to the intestine for each recording channel; channel 1, 1 cm proximal to the anastomosis; channel 2 and 3, within the bypass loop (oral and anal), channel 4, 1 cm distal to the anastomosis (Fig. 1). Clips were attached 2 cm apart to each other. Silk sutures were used to connect each clip to a force transducer (TST125C; Biopac Systems Inc., Santa Barbara, CA, USA). A single contraction is converted to a peak of wave by force transducer. Initial tension was set to below 5 mN to minimize local reflex stimulation of the bowel. Tension was monitored continuously using an MP150 interface and recorded on a PC running Acknowledge software ver. 3.2.6 (Biopac Systems Inc.). For the control mice, clips were attached to each other 2 cm apart and the contractile activity was measured in the same way.
The composition of the KRB was (in mM): NaCl 120.4; KCl 4.7; NaHCO3 15.5; Glucose 11.5; MgCl2 1.2; KH2PO4 1.2; CaCl2 2.5. The pH of the KRB was 7.3-7.4 when gassed with 97% O2-3% CO2 at 37.0 ± 0.5℃.
Contractile activity was analyzed from computer traces, and 4 to 5 representative MMCs from each animal were sampled for analysis. Amplitude (mN), duration of each contraction (seconds) and the interval between each contraction (seconds) were defined following the previously established parameters [8]. In brief, the amplitude and duration of each contraction was determined as the difference between peak tension and resting tension of each contraction, and the time between the half-maximal-amplitude points on the rising and falling phase, respectively (Fig. 2A). The interval between the contractions was determined by the time between the half-maximal-amplitude points on the rising phase of consecutive contractions (Fig. 2B). Contractions were quantified by calculations of the area above the baseline for each MMCs (area under the curve [AUC]; mN·sec/wave) by Acknowledge software ver. 3.2.6. The direction of MMC propagation was defined as either aboral, oral or bidirectional.
Data are expressed as means ± standard error of the mean. Parameters were compared between the control and operated model according to the recording channels with Mann-Whitney test. The proportion of propagation direction was compared between the control and operated model with chi-square test or Fisher's exact test. P < 0.05 was considered statistically significant.
Isolated segment of the ileum exhibited periodic contractions that propagated from the proximal to the distal regions of the tissue (Fig. 3). All the recording channels showed relatively regular contractions. Thirty-four MMCs were sampled at each recording channel and were analyzed. The amplitude, duration, interval and AUC of MMCs of each channel are shown in Table 1. Most of the MMCs were noted to propagate in an aboral direction (31/34, 91.1%). Only a few MMCs propagated orally (3/34, 8.9%). Bidirectionally propagating MMCs were not detected in the control animals.
Partial bypass model exhibited periodic contractions in channel 1, 2, 3 (proximal to the anastomosis, oral and anal side of the loop, respectively). In channel 4 (distal to the anastomosis), MMCs were either irregular or could not be detected and detailed analysis was not possible (Fig. 4A, B). Twenty MMCs were sampled at each recording channel and analyzed. The amplitude, duration, interval, and AUC of MMCs in each channel are shown in Table 1.
Significant changes were noted compared to the control, in every channel except channel 4. The amplitude was attenuated (P = 0.003, 0.009, 0.002 at channel 1, 2, 3, respectively), duration was shortened (P = 0.000, 0.000, 0.027 at channel 1, 2, 3, respectively) and the interval was also shortened (P = 0.003, 0.001, 0.005 at channel 1, 2, 3, respectively). The mean AUC for each MMC also decreased significantly at each of the recording sites (P = 0.000 at channel 1, 2, and 3).
Aborally-propagating MMCs (9/20, 45%) and orally-propagating MMCs (9/20, 45%) were equally noted, and bidirectional MMCs were also observed (2/20, 10%). Compared to the control, the proportion of orally-propagating MMCs was significantly increased (P = 0.002).
Similar to the 2-week model, periodic contraction occurred only in channel 1, 2, 3 (proximal to the anastomosis, oral and anal side of the loop; Fig. 4C, D). As in the 2-week model, detailed analysis of the MMCs was not possible for channel 4 (distal to the anastomosis). The mean amplitude, duration, interval and AUC of MMCs in each channel are shown in Table 1. Compared to the control, the amplitude was attenuated in every channel (P = 0.000, 0.000, 0.002 at channel 1, 2, 3, respectively). In contrast to the 2-week model, the duration of MMC was increased in channel 2 (P = 0.003), indicating that the duration of the MMCs recovered to the control state in the bypassed loop. Similarly, the interval of the MMCs recovered to the control level. Compared to the 2-week model, the amplitude in channel 1 was more attenuated (P = 0.000) but no significant changes were noted in the bypassed loop (channel 2, 3, P = not significant). The mean AUCs showed no significant change between 2- and 4-week model. Aborally-propagating MMCs occurred in 11/25 (44%), orally-propagating MMCs in 8/25 (32%), and bi-directionally propagating MMCs in 6/25 (24%). Compared to the 2-week model, the proportion of orally-propagating or bi-directionally propagating MMCs did not change significantly (P = 0.422).
Periodic contractions were observed in channel 1, 2, 3 (proximal to the anastomosis, oral and anal side of the loop), but the contractions in channel 1 did not propagate to channel 2 (Fig. 5A), and the bypass loop had independent MMCs not coordinated to the proximal segment. In channel 4 (distal to the anastomosis), no definite MMCs could be detected. Twenty MMCs were sampled at each recording channel and analyzed. The mean amplitude, duration, interval and AUC of MMCs in each channel are shown in Table 1. Compared to the control, the amplitude was attenuated in channel 1, 2, and 3 (P = 0.002, 0.000, 0.000, respectively) and the duration was shortened in channel 1 (P = 0.044). The mean AUC decreased significantly at channel 1, 2, and 3 (P = 0.000, 0.000, 0.007, respectively). Nineteen of twenty (95%) MMCs propagated aborally, which was similar to the control state, and 1 (5%) simultaneous MMC was observed within the loop. Neither orally-propagating nor bidirectional MMCs were observed.
Periodic contractions were noted only in channel 2 and 3 (oral and anal side of the loop). There were two different patterns of MMCs in the total bypass 4-week model: independently pacing MMCs without temporal relationship within the loop (n = 3, Fig. 5B), and irregular, small amplitude contractions (n = 2, Fig. 5C). The mean amplitude, duration, interval and AUC of MMCs in each channel are shown in Table 1. Compared to the control, the amplitude was attenuated in channel 2 and 3 (P = 0.000, 0.000, respectively), the duration was shortened in channel 2 (P = 0.004), and the interval was shortened in channels 2 and 3 (P = 0.001, 0.034). Compared to the 2-week model, the interval was shortened more within the bypass loop (P = 0.001, 0.003). The direction of propagation could not be defined, though, as the contractions within the loop were temporarily dissociated from each other. The mean AUC showed no significant changes between the 2-week and 4-week model.
This study was performed in order to investigate the mechanism underlying the development of the blind loop syndrome through the changes in MMC. Historically, MMC has been a field of interest for physiologists studying gastrointestinal motility, and most of the physiologic properties of MMC have been clarified by using native animal bowel without any surgical manipulations, usually through ex-vivo observations. There have been only a few studies reporting physiological motility changes after surgical manipulation on gastrointestinal tract. Chang et al. [9] reported the loss of interstitial cell of Cajal (ICC) and slow wave in murine small bowel obstruction model. Yanagida et al. [10] showed the loss of ICC and slow wave, and decrease in contractile force 5 hours after intestinal transection and re-anastomosis in mouse, which were recovered in 24 hours. However, none of these two previous studies dealt with the MMC in their animal model. To our knowledge, the present study is the first one demonstrating the significant changes in MMC after surgical manipulation of the mouse intestine.
It has been assumed that the bypass loop acts as a reservoir, and stagnation with resultant bacterial overgrowth inevitably follows. Vantrappen et al. [11] assumed that decreased or absent inter-digestive motor complex (which is equivalent to MMC in mouse intestine) might be related to the intraluminal bacterial overgrowth in human. However, the proof for such phenomenon was lacking. In this study, at least in cases of side-to-side anastomosis for partial bypass in mouse, we have shown that the changes in the direction of propagation of the MMCs from the normal aboral to oral propagation within the bypass loop might be responsible for stagnation of the bowel contents. As time progressed after partial bypass, the changes in the direction of propagation of the MMCs became more prominent. The reason for the changes in the direction of the MMCs cannot be clearly explained, but we speculate that intact, undisturbed continuity of the intestinal tract is necessary for orderly aboral propagation of the MMCs. The decreased AUC following the bypass operation implies the significant loss of contractile activity in the bypassed loop, which might explain the progressive dilatation of the bypass loop observed in blind loop syndrome.
In the total bypass model, where the proximal portion of the bypassed loop was occluded, the MMCs in channel 1 (proximal to the anastomosis) did not propagate to channel 2 (oral side of the bypassed loop). Our previous study indicated that partial obstruction in the mouse ileum blocked the propagation of the MMCs at the obstruction site and MMCs distal to the obstruction paced at a rhythm different from that proximal to the obstruction. This indicates that a site distal to the obstruction serves as a new pacemaker for MMCs [12]. We speculate that a similar mechanism might explain the different rhythm between channel 1 (proximal to the anastomosis) and channel 2 (oral side of the bypassed loop) in total bypass model. The site distal to the obstruction (i.e., proximal loop) became the new pacemaker and served to provide one-way (aboral) propagation within the loop. This contrasts with the partial bypass model, where passage is maintained and MMCs are sequentially related between the segment proximal to the anastomosis (channel 1) and bypass loop (channel 2, 3), irrespective of the direction of propagation. As the duration of total bypass became longer, MMCs within the loop lost its temporal relationship and paced independently. Moreover, in some cases, meaningful MMCs disappeared and irregular contractions remained. This might reflect the loss of physiological role of MMCs (periodically cleansing the bowel) secondary to the complete disuse of the bypass loop in a total bypass model.
Interestingly in our bypass model, the MMCs were consistently irregular or could not be detected in the segment distal to the anastomosis (channel 4). However, using pellets, we could demonstrate that passage was maintained at that segment (data not shown). And since the mice survived after 2 to 4 weeks after bypass operation, we could assume that passage of food material was maintained. We do not have a clear explanation for this phenomenon. A previous study, which used a transection and re-anastomosis model in a guinea pig, demonstrated that the MMCs were unable to pass through the suture line [13]. Also, the neuronal integrity is known to be essential for the propagation of the MMCs [14]. Therefore, due to the disrupted neuronal integrity by the anastomosis for bypass, we might assume that the MMCs at proximal segment could not pass through the anastomosis to the distal segment. Another possible explanation is the collision of the neuronal signals from the proximal segment and the bypass loop at the anastomosis site, causing offset of signals necessary to generate MMCs. In our experiment, MMCs could be detected in sites more distal to the anastomosis, indicating that a new pacemaker site for MMCs appeared (data not shown).
It is known that initiation and propagation of MMCs are dependent on the intrinsic enteric nervous system; MMCs continue to occur after extrinsic denervation and are blocked by nerve conduction blocker such as tetrodotoxin [15]. After 4 weeks of total bypass, MMCs proximal to the anastomosis (channel 1) disappeared and occasional uncoordinated contractions remained, which were thought to be the result of local reflex rather than true MMCs. The only possible explanation is that intact undisturbed continuity of the intestinal tract with its intact neuronal circuit is necessary for the presence of MMCs.
Slow waves, which arise from the ICC, are another significant component of the intestinal motility especially in the mouse ileum [16]. In order to determine whether changes in the slow waves were present in the bypass loop, we performed conventional intracellular recordings and c-kit immunohistochemistry. Our results indicated that slow wave and ICC remained unchanged in the bypass loop (data not shown). Thus, we could conclude that the slow wave was not responsible for the development of blind loop syndrome.
This study has limitations in that the experiments were conducted in ex-vivo conditions. There may be doubts on the relevance of the ex-vivo motor function to the in-vivo situations. However, although some of the classic works on the gastrointestinal motility had been performed with animals in-vivo [4,5], most of the studies on mouse MMC have been performed with reproducibility in ex-vivo conditions [14,15]. Certainly, the intestine in our model may be autonomically denervated extrinsically, but MMC is mainly dependent on intrinsic nervous system which remains intact in the undisturbed bowel segment ex-vivo. Moreover, with the explosive growth in both transgenic and genomic technology, the mouse is accepted as a useful model system for investigating motility of the mammalian gastrointestinal tract [7]. Also, in each bypass model, the MMCs showed constant changes compared to the control state and therefore extrapolation from the ex-vivo results to the in-vivo situation may be reasonable.
In summary, after side-to-side partial bypass anastomosis in mouse ileum, changes in the MMCs, especially in the direction and contractile activity within the bypass loop were noted. These changes may account for the development of stasis of luminal contents and thereby blind loop syndrome. In the total bypass model, the propagation of MMCs was maintained for a period of time. This implies the beneficial role of side-to-side anastomosis with proximal loop ligation or Roux-en-Y type of bypass, and should be considered when bypass surgery has to be performed.
Figures and Tables
Fig. 1
Schematic drawing and operative photograph of the partial bypass model (A) and total bypass model (B). In contrast to the partial bypass model, there were no luminal contents within the bypass loop in total bypass model. As such, the diameter of the bypassed loop tended to be greater in the partial bypass model compared to the total bypass model.

Fig. 2
Definition of parameters for migrating motor complex. (A) Amplitude and duration of each wave and (B) interval between each wave.
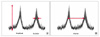
Fig. 3
Representative traces of migrating motor complexes (MMCs) recorded from the control mice. Dotted lines show the aboral propagation of the MMCs.
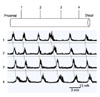
Fig. 4
Representative traces of the migrating motor complexes recorded from the partial bypass for 2 weeks (A, B) and for 4 weeks (C, D). Dotted lines show oral (A, C) or bidirectional (B, D) propagation.
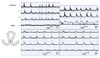
Fig. 5
Representative traces of the migrating motor complexes (MMCs) recorded from the total bypass for 2 weeks (A) and for 4 weeks (B, C). (A) MMCs proximal to the anastomosis have no temporal relationship with those within the bypass loop. (B) MMCs lost temporal relationship and (C) regular MMCs were lost within the bypass loop.
ACKNOWLEDGMENTS
This study was supported by the Grant of Clinical Research Institute, Seoul National University Hospital, Seoul, Korea (No. 04-2007-074).
Notes
References
1. Botsford TW, Gazzaniga AB. Blind pouch syndrome. A complication of side to side intestinal anastomosis. Am J Surg. 1967. 113:486–490.
2. Adachi Y, Matsushima T, Mori M, Sugimachi K, Oiwa T. Blind loop syndrome: multiple ileal ulcers following side-to-side anastomosis. Pathology. 1993. 25:402–404.
3. Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992. 216:269–278.
4. Wingate DL. Backwards and forwards with the migrating complex. Dig Dis Sci. 1981. 26:641–666.
5. Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985. 89:894–913.
6. Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am J Dig Dis. 1973. 18:477–488.
7. Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000. 84:162–168.
8. Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997. 9:99–107.
9. Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001. 536(Pt 2):555–568.
10. Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004. 127:1748–1759.
11. Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977. 59:1158–1166.
12. Moon SH, Oh HK, Ryoo S, Choe EK, Moon JS, Park KJ. Changes in migrating motor complex after bowel obstruction in the murine ileum. J Korean Soc Coloproctol. 2010. 26:171–178.
13. Galligan JJ, Furness JB, Costa M. Migration of the myoelectric complex after interruption of the myenteric plexus: intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology. 1989. 97:1135–1146.
14. Powell AK, O'brien SD, Fida R, Bywater RA. Neural integrity is essential for the propagation of colonic migrating motor complexes in the mouse. Neurogastroenterol Motil. 2002. 14:495–504.
15. Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol. 2001. 1:604–610.
16. Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006. 290:C1411–C1427.




 Citation
Citation Print
Print



 XML Download
XML Download