Abstract
Purpose
Methods
Results
Conclusion
Figures and Tables
Fig. 1
The anatomic area used for the assessment of dis semination. Inferior (A, B) and frontal views (C, D) in male and female; limited grade refers to gangrene localized in the “Y” area of the perineum, scrotum and penis, vulva, peri anal, or inguinal region. Extended grade disease re fers to extension of the disease beyond these areas.
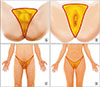
Fig. 3
A case of Fournier gangrene. A 60-year-old male with no past medical history was referred to the Department of Plastic and Reconstructive Surgery for wound reconstruction. Necrotizing fasciitis spread to the perianal area and scrotum, defined as limited grade. (A) After debridement, testicles were exposed. (B) Reconstruction with medial circumflex femoral artery perforator flap. (C) Immediate postoperative view. (D) A photograph obtained 2 weeks postoperatively.
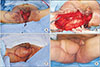
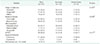
Table 2
Clinical data based on vital signs and laboratory values at initial presentation

Values are presented as number (%) or mean ± standard deviation.
SIRS, systemic inflammatory response syndrome; CRP, C-reactive protein; FGSI, Fournier Gangrene Severity Index; LRINEC, laboratory risk indicator for necrotizing soft tissue infection.
a)t-test or Wilcoxon rank sum test. b)Chi-square test or Fisher exact test.
Table 6
Multiple logistic regression analysis of variables affecting mortality and flap reconstruction

Reference categories for categorical variables in the column ‘variables’ listed in parentheses.
CI, confidence interval; CKD, chronic kidney disease; SIRS, systemic inflammatory response syndrome; FGSI, Fournier Gangrene Severity Index; LRINEC, laboratory risk indicator for necrotizing soft tissue infection.
Table 7
Logistic and linear regression analysis of factors determining intensive care unit (ICU) stay over 7 days and total hospital stay

Reference categories for categorical variables in the column ‘variables’ listed in parentheses.
CI, confidence interval; CKD, chronic kidney disease; SIRS, systemic inflammatory response syndrome; FGSI, Fournier Gangrene Severity Index; LRINEC, laboratory risk indicator for necrotizing soft tissue infection.
a)Log-linear regression model.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download