Abstract
Purpose
Methods
Results
Conclusion
ACKNOWLEDGEMENTS
References
Fig. 1
Study groups. Group I: Non-AKI & SCD (n = 97); group II: Non-AKI & ECD (n = 15); group III: AKI & SCD (n = 52); group IV: AKI & ECD (n = 38); AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor.
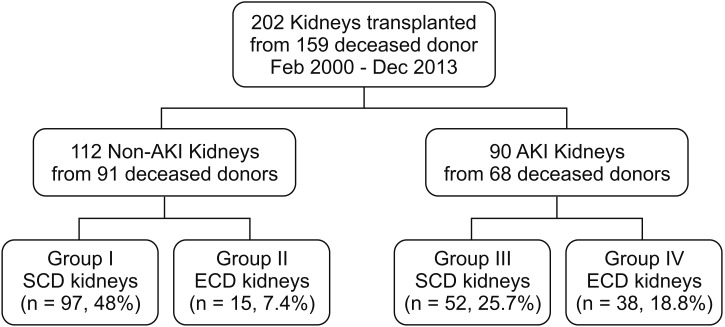
Fig. 2
Change in graft function. AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor; MDRD, modification of diet in renal disease; eGFR, estimated glomerular filtration rate; KT, kidney transplantation.
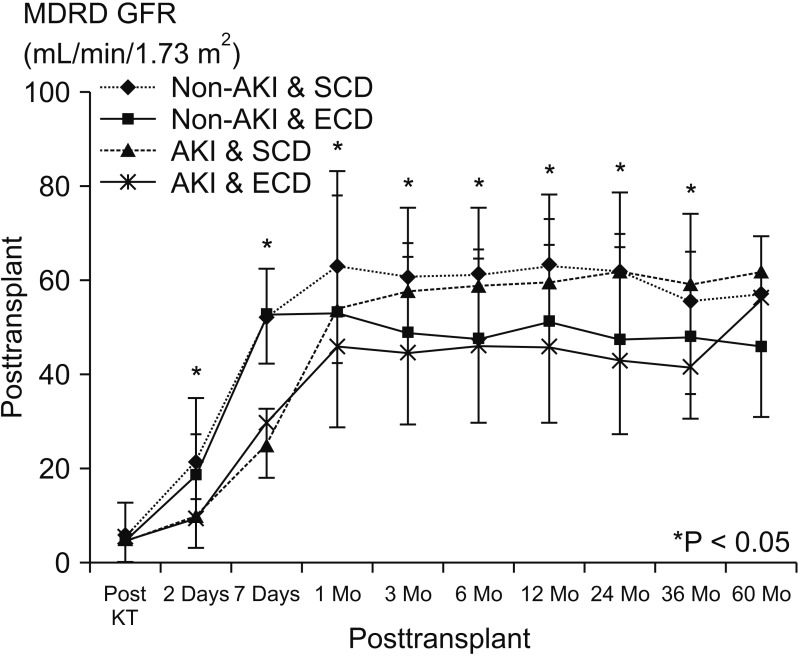
Fig. 3
Graft (A, P = 0.074) and patient survival (B, P = 0.090) among the 4 groups. AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor.
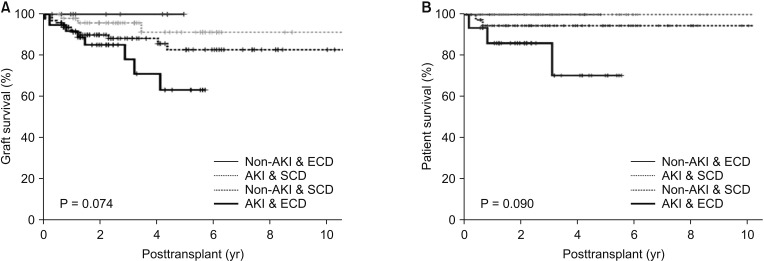
Fig. 4
Comparing graft survival (P = 0.024) between group IV and the others (groups I, II, and III). Group I: Non-AKI & SCD (n = 97); group II: Non-AKI & ECD (n = 15); group III: AKI & SCD (n = 52); group IV: AKI & ECD (n = 38); AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor.
Table 1
Donor characteristics by group (n = 159)
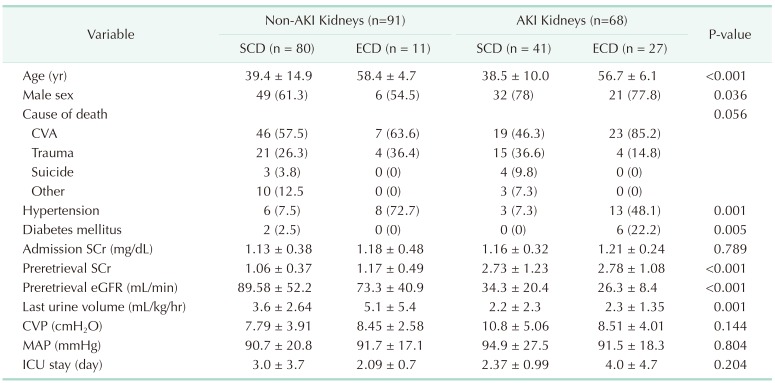
Values are presented as mean ± standard deviation or number (%).
AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor; CVA, cerebrovascular accident; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; CVP, central venous pressure; MAP, mean arterial pressure; ICU, intensive care unit.
Table 2
Recipient characteristics by group (No. of recipients = 20)
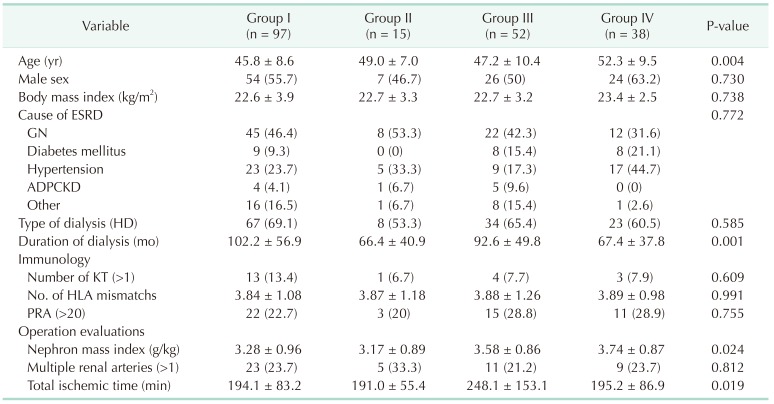
Values are presented as mean ± standard deviation or number (%).
Group I: Non-AKI & SCD (n = 97); group II: Non-AKI & ECD (n = 15); group III: AKI & SCD (n = 52); group IV: AKI & ECD (n = 38); AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor; ESRD, end-stage renal disease; GN, glomerulonephritis; ADPCKD, autosomal dominant polycystic kidney disease; HD, hemodialysis; KT, kidney transplantation; HLA, human leukocyte antigen; PRA, panel-reactive antibody.
Table 3
Change in the graft function with time (No. of recipients = 202)
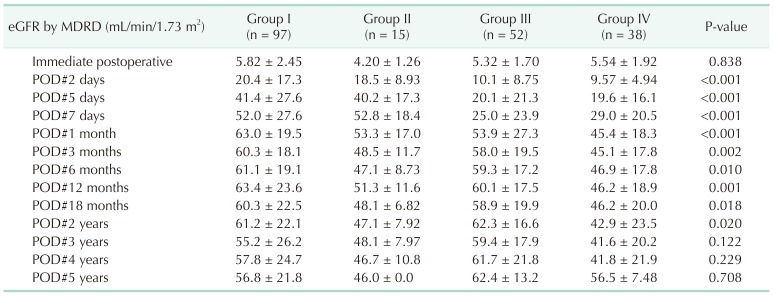
Values are presented as mean ± standard deviation.
Group I: Non-AKI & SCD (n = 97); group II: Non-AKI & ECD (n = 15); group III: AKI & SCD (n = 52); group IV: AKI & ECD (n = 38); AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; POD, postoperative day.
Table 4
Outcomes by group
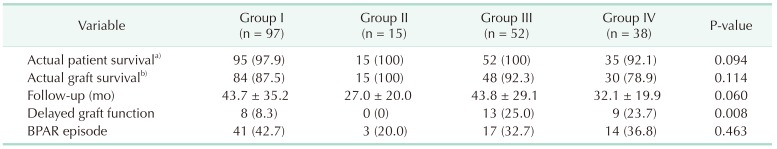
Values are presented as number (%) or mean ± standard deviation.
Group I: Non-AKI & SCD (n = 97); group II: Non-AKI & ECD (n = 15); group III: AKI & SCD (n = 52); group IV: AKI & ECD (n = 38); AKI, acute kidney injury; SCD, standard criteria donor; ECD, expanded criteria donor; BPAR, biopsy proven acute rejection.
a, b)During the follow-up period.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download