Abstract
Purpose
Recently, the American Thyroid Association (ATA) dynamic risk stratification (DRS) has been verified to be more valuable than the static anatomical staging system for predicting prognosis in patients with differentiated thyroid carcinoma (DTC). The purpose of this retrospective study was to compare the clinical usefulness of DRS, which is based on the response to initial treatment, with that of ATA initial risk stratification in pediatric patients.
Methods
A total of 144 pediatric patients underwent thyroid operation from August 1982 to December 2013 at Yonsei University Hospital (Seoul, Korea). Among them, 128 patients with complete clinical data were enrolled in this study. Clinicopathologic features and surgical outcomes were retrospectively analyzed by medical chart review. The mean follow-up duration was 11.5 years.
Results
The mean tumor size was 2.1 cm; 80.4% of patients were diagnosed with conventional papillary thyroid carcinoma, and 7.0% of patients were diagnosed with follicular thyroid carcinoma. Low-risk patients had the highest probability of an excellent response to initial treatment (66.6%). High-risk patients had the highest probability of a structural incomplete response (100%) and the lowest probability of an excellent response (11.1%). The ATA risk stratification and the DRS system were independent risk factors for disease-free survival (DFS) (P = 0.041 and P < 0.001, respectively).
The incidence and prevalence of differentiated thyroid carcinoma (DTC) have been rising significantly over the last 2 decades [123]. DTC is the most common endocrine carcinoma in pediatric patients and accounts for 2%–10% of all pediatric carcinomas [456]. The distinctive feature of DTC in pediatric patients is its aggressiveness, with more lymph node (LN) metastasis, distant metastasis, and extrathyroidal extension (ETE) [7891011]. At the same time, the mortality rate in pediatric patients is extremely low [12131415].
The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system is widely used for predicting the prognosis of DTC [16]. However, the AJCC/UICC TNM staging system has a limitation with regard to the assessment of DTC in pediatric patients. Unlike with adults, the disease in pediatric patients is classified into only 2 stages due to the age cutoff: stage I without distant metastasis and stage II with distant metastasis.
In the American Thyroid Association ( ATA) guidelines newly released in 2015, pediatric patients with DTC were reclassified into 3 groups: low, intermediate, and high risk according to extent of tumor and nodal status [17]. However, this risk stratification system is still inadequate for predicting accurate prognoses in pediatric patients.
A previous study suggested a dynamic risk stratification (DRS) system that was based on response to initial treatment [18]. Patients with DTC were restratified according to suppressed and stimulated thyroglobulin (Tg) levels, Tg antibody (TgAb) level, and imaging modalities, including neck ultrasonography and whole body scan (WBS) during follow-up. This study reported that the DRS system provided more accurate prognostic information. However, to the best of our knowledge, there have been few studies investigating the use of the DRS system for pediatric patients with DTC.
The purpose of this study was to identify the clinical value of the ATA risk stratification system in pediatric patients with DTC. We also compared the clinical usefulness of the DRS system with that of ATA initial risk stratification.
A total of 144 pediatric patients with DTC who had undergone thyroid operations from August 1982 to December 2013 were retrospectively reviewed at Yonsei University Hospital (Seoul, Korea). Sixteen patients were excluded because they had inadequate follow-up data; thus, 128 patients were analyzed by complete review of medical charts and pathology reports. Among them, 40 (31.2%) had undergone bilateral total thyroidectomy (BTT), and 43 (33.6%) had undergone BTT with therapeutic modified radical neck dissection (mRND). These patients received subsequent radioactive iodine (RAI) ablation. The mean follow-up duration was 11.5 years (range, 2.1–29.8 years). This study was approved by the at Yonsei University Hospital Institutional Review Board (4-2015-1160).
The management protocol followed the ATA management guidelines [17]. Tg and TgAb concentrations were regularly measured, and physical examinations, neck ultrasonography and thyroid function tests were performed at 3 and 6 months and annually thereafter. Postoperative RAI treatment was performed 4 to 6 weeks after operation, and WBSs were performed 5 to 7 days after RAI ablation. All patients were prescribed a suppressive dose of L-thyroxine (thyroidstimulating hormone [TSH] < 0.05 mIU/L). Patients who had clinical suspicion of recurrence of disease or distant metastasis were evaluated by additional diagnostic imaging, including computed tomography, positron emission tomography/computed tomography, and/or RAI WBS. To confirm the recurrence of disease, ultrasound-guided fine needle aspiration biopsy was performed in addition to imaging modalities.
The 2015 ATA risk stratification system for pediatric patients was applied to the study subjects, dividing them into low, intermediate, and high-risk groups [17]. The low-risk group was defined as pediatric patients with DTC grossly confined to the thyroid (AJCC/UICC TNM staging system T1 to T3) with clinical N0/Nx disease or with pathologic N1a micrometastases (number of LNs ≤ 5 and largest dimension of metastatic foci < 0.2 cm). The intermediate-risk group was defined as pediatric patients with DTC accompanied with pathologic N1a metastases (number of LNs > 5 or largest dimension of metastatic foci ≥ 0.2 cm) or minimal N1b metastases (number of LNs ≤ 10 and largest dimension of metastatic foci < 3 cm). The high-risk group was defined as pediatric patients with DTC with pathologic N1b metastases (number of LNs > 10 or largest dimension of metastatic foci ≥ 3 cm), locally invasive disease (T4), or distant metastasis.
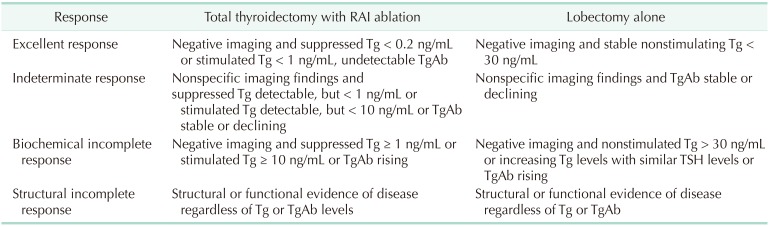
The DRS system classified pediatric patients into 4 groups, as shown in previous studies: excellent response, indeterminate response, biochemical incomplete response, and structural incomplete response [18192021]. The best response to the initial treatment was evaluated between 1 and 2 years after diagnosis of DTC with Tg level, TgAb level, and imaging findings. The definition of DRS classification according to the extent of surgery is shown in Table 1.
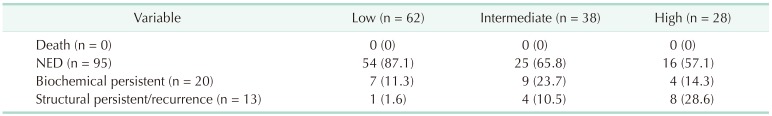
Patients with negative stimulated Tg, negative TgAb, and no evidence of structural disease at the final follow-up were defined as having no evidence of disease (NED). Biochemical-persistent disease in patients who underwent total thyroidectomy with RAI ablation was defined as negative imaging findings with suppressed Tg > 1 ng/mL or increasing TgAb level; in patients who underwent lobectomy, this was defined as negative imaging findings with nonstimulated Tg > 30 ng/mL or increasing Tg level with similar TSH level or increasing TgAb. Structural persistent/recurrent disease was defined as pathologically or cytologically verified malignancy and/or metastasis by imaging modalities regardless of Tg or TgAb level [20].
Continuous, quantitative variables are provided as mean with standard deviation. Categorical and qualitative variables are reported as number with percentage. Multivariate Cox regression analysis was performed to identify the independent risk factors of DFS. DFS was compared by Kaplan-Meier analysis with log-rank test between groups. The proportion of variance explained (PVE) value was calculated with the Cox regression model, using the following equation:
PVE = 1 − exp(-G2/n)
where G2 is the maximum likelihood ratio from the Cox regression model, and n is the total number of patients [22]. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated, and 2-sided P-values of <0.05 were regarded as statistically significant. All statistical analyses were performed using R package version 3.1.3 (http://www.R-project.org).
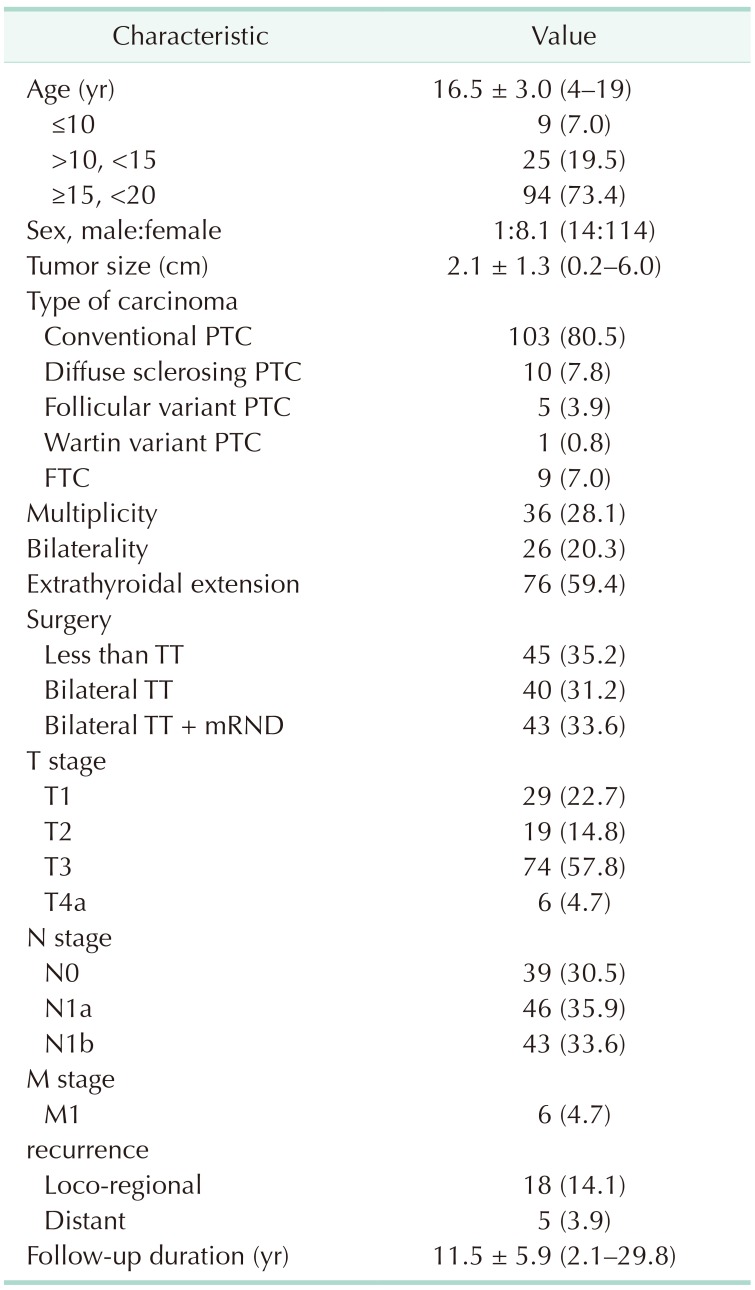
Table 2 provides the baseline clinicopathologic characteristics of the 128 pediatric patients with DTC. The mean age of study patients was 16.5 years (range, 4–19 years); among them, 9 (7.0%) were younger than 10 years. A total of 114 patients (89.1%) were female, and the mean tumor size was 2.1 cm (range, 0.2–6.0 cm). A total of 103 patients (80.5%) were diagnosed with conventional papillary thyroid carcinoma (PTC), and 9 patients (7.0%) were diagnosed with follicular thyroid carcinoma (FTC). The remaining patients (12.5%) were diagnosed with other types of PTC. Multiplicity and bilaterality of carcinoma were found in 36 (28.1%) and 26 patients (20.3%), respectively. Seventy-six patients (59.4%) were identified with ETE. Forty patients (31.2%) underwent BTT, and 43 patients (33.6%) underwent BTT with therapeutic mRND due to N1b nodes. The numbers of patients with T1, T2, T3, and T4a were 29 (22.7%), 19 (14.8%), 74 (57.8%), and 6 (4.7%), respectively. Forty-six patients (35.9%) had cervical LN metastasis, and 43 patients (33.6%) had lateral LN metastasis. Only 6 patients (4.7%) were diagnosed as having distal metastasis of the lung at the same time as diagnosis of thyroid carcinoma. Loco-regional recurrence was detected in 18 patients (14.1 %), and recurrence with distant metastasis to the lung was detected in 5 patients (3.9%). The mean follow-up duration was 11.5 years (range, 2.1–29.8 years).
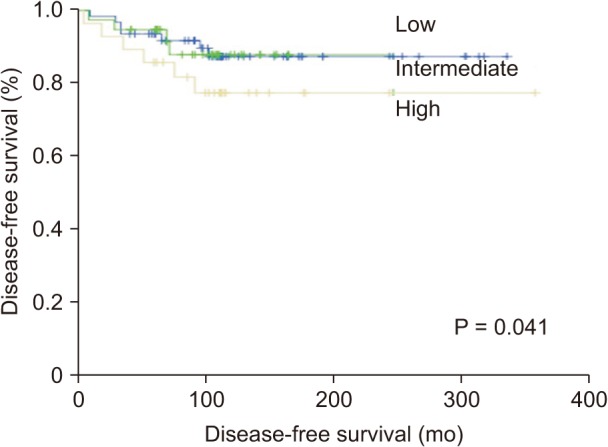
Using the ATA risk stratification system, 62 patients (48.4%) were classified into the low-risk group, 38 patients (29.7%) were classified into the intermediate-risk group, and 28 patients (21.9%) were classified into the high-risk group. Table 3 shows the clinical outcomes according to the ATA risk stratification system. Most of the patients (87.1%) in the low-risk group had NED, and only 1 patient (1.6%) was diagnosed with structural persistent/recurrent disease. On the other hand, 8 patients (28.6%) in the high-risk group were diagnosed with structural persistent/recurrent disease. The DFS of the ATA risk stratification system were significantly different (P = 0.041) (Fig. 1).
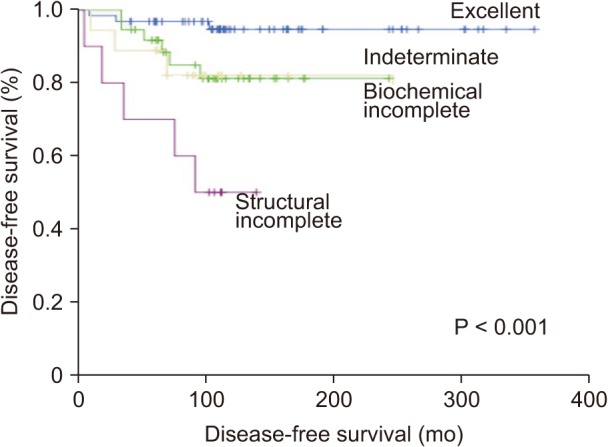
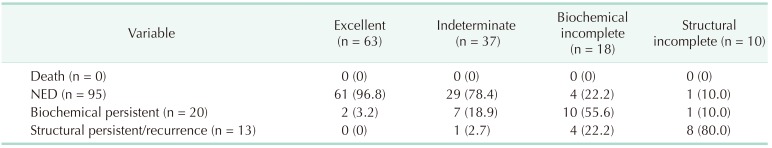
Using the DRS system, the patients were reclassified into 4 groups: excellent response, 63 (49.2%); indeterminate response, 37 (28.9%); biochemical incomplete response, 18 (14.1%); and structural incomplete response, 10 (7.8%). Table 4 presents the clinical outcomes according to the DRS system. Sixty-one patients (96.8%) in the excellent response group had NED, and only 2 patients (3.2%) had biochemical persistent disease. The majority of patients (78.4%) in the indeterminate group had NED, while 7 patients (18.9%) and 1 patient (2.7%) were verified as having biochemical persistent and structural persistent/recurrent disease, respectively. On the other hand, slightly more than half of the patients (55.6%) in the biochemical incomplete group were diagnosed with biochemical persistent disease, and 4 patients (22.2 %) were diagnosed with structural persistent/recurrent disease. Eight patients (80%) in the structural incomplete response group were diagnosed with structural persistent/recurrent disease, and only 1 patient (10%) had NED. The Kaplan-Meier survival analysis showed statistically significant differences among the response groups with the DRS system (P < 0.001) (Fig. 2).
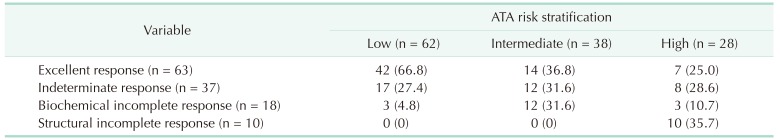
The correlation between the ATA risk stratification system and the DRS system is shown in Table 5. Of the patients who had excellent responses at initial evaluation after initial therapy, 42 (66.6%) were in the low-risk group, while 7 (11.1%) were in the high-risk group. Twelve patients (66.6%) with biochemical incomplete response were in the intermediate-risk group, and all patients with structural incomplete response were in the high-risk group.
To compare the predictive accuracy of prognosis between the ATA risk stratification system and the DRS system, the PVE was calculated. The PVE value of the DRS system was 54.9%, which was higher than that of the ATA risk stratification system, 29.8%.
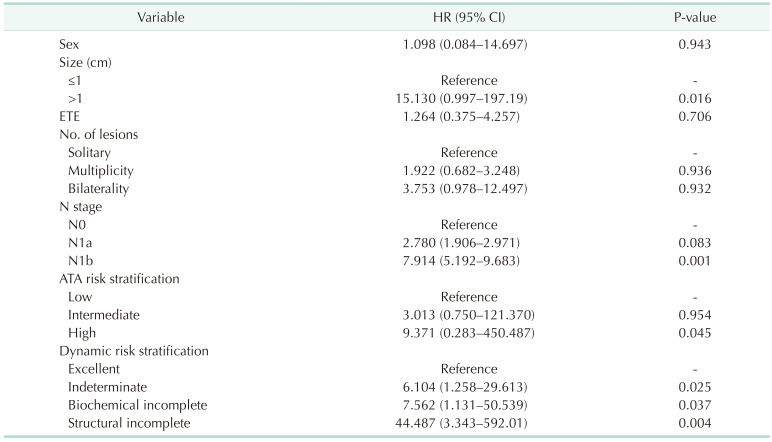
To determine the independent risk factors associated with DFS, a multivariate analysis was performed (Table 6). Tumor size over 1 cm (HR, 15.130; 95% CI, 0.997–197.19; P = 0.016) and N1b stage (HR, 7.914; 95% CI, 5.192–9.683; P = 0.001) were confirmed as significant predictors for recurrence. In the ATA risk stratification system, the risk of recurrence was significantly higher in the high-risk group (HR, 9.371; 95% CI, 0.283–450.487; P = 0.045) than in the low-risk group. The risk of recurrence in the DRS system was significantly higher in all other groups than in the excellent response group: indeterminate group (HR, 6.104; 95% CI, 1.258–29.613; P = 0.025), biochemical incomplete group (HR, 7.562; 95% CI, 1.131–50.539; P = 0.037), and structural incomplete group (HR, 44.487; 95% CI, 3.343–592.01; P = 0.004).
The ATA risk stratification system released in 2015 is regarded as an appropriate method for prediction of recurrent or persistent disease after initial treatment in pediatric patients with DTC [17]. In the ATA system, pediatric patients are classified into 3 risk groups. However, several studies have reported that the ATA risk stratification system is inappropriate to predict prognosis in pediatric patients with DTC [1923]. The present study was designed to compare the clinical usefulness of the ATA risk stratification system with that of the DRS system, which is based on the initial treatment response as measured by serum Tg and TgAb levels and imaging findings. Serum Tg level is used as a tumor marker for patients with DTC after BTT. However, false-negative results could result from TgAb interference with Tg measurements [24].
The clinical features of DTC are different between pediatric and adult patients. LN metastasis occurs in 60% to 80% of pediatric patients with DTC, and lung metastasis occurs in 10% to 20% [25]. In our study, 89 (69.5%) and 6 patients (4.7%) were diagnosed as having LN and lung metastasis, respectively. However, in spite of aggressive disease, pediatric patients with DTC have a lower mortality rate than adult patients. Even though the evidence for the reasons for this difference is unclear, long-term prognosis of pediatric patients is excellent [2627].
We recategorized pediatric patients into 4 groups according to response to initial treatment: excellent, indeterminate, biochemical incomplete, and structural incomplete. During the follow-up period, 42 patients (66.8%) in the low-risk group were recategorized into the excellent response group, and 7 patients (25.0%) in the high-risk group were recategorized into the excellent response group. Of the patients in the excellent response group, 96.8% had NED, but only 2 (3.2%) were diagnosed with structural persistent/recurrent disease. Twelve of the 38 patients (31.6%) in the intermediate-risk group were reclassified into the biochemical incomplete response group. The number of patients reclassified according to the DRS system was significant considering the possibility (77.8%) of biochemical persistent or structural persistent/recurrent disease. In the high-risk group, 10 patients (35.7%) were reclassified into the structural incomplete response group, and 90% of the patients in the structural incomplete response group were diagnosed as having biochemical persistent or structural persistent/recurrent disease. The DRS system could provide a wider variety of information about patient disease status in order to modify the management strategy or to better determine the treatment plan for pediatric patients with DTC.
The ATA guidelines recommend total thyroidectomy for pediatric patients with DTC, because they have an increased incidence of bilateral and multifocal disease [17]. Total thyroidectomy could guarantee RAI ablation and the use of Tg as a tumor marker. Eighty-three patients (64.8%) underwent total thyroidectomy in this present study. However, 45 patients (35.2%) underwent unilateral thyroidectomy due to limited disease in the unilateral thyroid.
In the multivariate analysis, the results showed that the high-risk group had a significantly higher risk of recurrence compared with the low-risk group (P = 0.045), but the intermediate-risk group was similar to the low-risk group (P = 0.954) according to the ATA risk stratification system. On the other hand, the indeterminate, biochemical incomplete, and structural incomplete response groups were at significantly higher risk of recurrence compared with the excellent group (P = 0.025, P = 0.037, and P = 0.004, respectively) according to the DRS system. According to this analysis, we can infer that the DRS system is more useful to predict recurrence in pediatric patients with DTC. Similar to previous studies, our results also showed that tumor size (>1 cm) and N stage (N1b) were predictors for recurrence [2829].
In addition, considering the PVE value, the DRS system has a clinical benefit for more precise predictability of DFS in pediatric patients with DTC. The results of this research support the idea that the DRS system is more useful for predicting persistent/recurrent disease compared with the ATA risk stratification system in pediatric patients with DTC.
Several studies have been performed to investigate the accuracy with which systems predict prognosis in pediatric patients with DTC [615192330]. Lazar et al. [23] reported that their scoring system based on response to initial treatment was suitable for predicting the risk of persistent/recurrent disease in pediatric patients with DTC. Zanella et al. [30] found that postoperative stimulated Tg had high accuracy for prediction of persistent disease in pediatric patients with DTC. However, prognostic factors of pediatric patients with DTC are still controversial.
There are several limitations in the present study. Firstly, this study was retrospective in nature. Secondly, the study population included only 128 pediatric patients, which was a relatively small number of patients, and had a relatively short follow-up period (11.5 ± 5.9 years). Thirdly, there might have been selection bias as all patients were from a single tertiary institution. Finally, the mean age was 16.5 years (range, 4–19 years): 94 patients (73.5%) were older than 15 years, and only 9 patients (7.0%) were younger than 10 years.
The most important strength of this study is that all patients in our study were followed up with the same protocol, including type of surgery, RAI ablation protocol, degree of TSH suppression, and use of imaging modalities.
In conclusion, the DRS system is likely to enhance the predictability of prognosis with ATA risk stratification in pediatric patients with DTC. Further studies are required to determine whether DRS with adjusted response to the initial therapy regimen is useful in clinical practice.
References
1. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–450. PMID: 26987395.

2. Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009; 18:784–791. PMID: 19240234.

3. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014; 140:317–322. PMID: 24557566.

4. Collini P, Massimino M, Leite SF, Mattavelli F, Seregni E, Zucchini N, et al. Papillary thyroid carcinoma of childhood and adolescence: a 30-year experience at the Istituto Nazionale Tumori in Milan. Pediatr Blood Cancer. 2006; 46:300–306. PMID: 16047353.

5. Park S, Jeong JS, Ryu HR, Lee CR, Park JH, Kang SW, et al. Differentiated thyroid carcinoma of children and adolescents: 27-year experience in the yonsei university health system. J Korean Med Sci. 2013; 28:693–699. PMID: 23678260.

6. Pires BP, Alves PA Jr, Bordallo MA, Bulzico DA, Lopes FP, Farias T, et al. Prognostic factors for early and long-term remission in pediatric differentiated thyroid carcinoma: the role of sex, age, clinical presentation, and the newly proposed American Thyroid Association Risk Stratification System. Thyroid. 2016; 26:1480–1487. PMID: 27540892.

7. Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006; 243:525–532. PMID: 16552205.

8. Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf). 1998; 49:619–628. PMID: 10197078.

9. Schlumberger M, De Vathaire F, Travagli JP, Vassal G, Lemerle J, Parmentier C, et al. Differentiated thyroid carcinoma in childhood: long term follow-up of 72 patients. J Clin Endocrinol Metab. 1987; 65:1088–1094. PMID: 3680475.

10. Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr. 2009; 154:708–714. PMID: 19167722.

11. Nam KH, Lim CY, Lee J, Chang HS, Chung WY, Choi SH, et al. Differentiated thyroid carcinoma in patients less than 20 years of age at diagnosis: clinicopathologic characteristics and prognostic factors. J Korean Surg Soc. 2005; 69:443–449.
12. Jarzab B, Handkiewicz Junak D, Wloch J, Kalemba B, Roskosz J, Kukulska A, et al. Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med. 2000; 27:833–841. PMID: 10952495.
13. Papendieck P, Gruneiro-Papendieck L, Venara M, Acha O, Cozzani H, Mateos F, et al. Differentiated thyroid cancer in children: prevalence and predictors in a large cohort with thyroid nodules followed prospectively. J Pediatr. 2015; 167:199–201. PMID: 26117640.

14. Alzahrani AS, Alkhafaji D, Tuli M, Al-Hindi H, Sadiq BB. Comparison of differentiated thyroid cancer in children and adolescents (≤20 years) with young adults. Clin Endocrinol (Oxf). 2016; 84:571–577. PMID: 26118454.
15. Markovina S, Grigsby PW, Schwarz JK, DeWees T, Moley JF, Siegel BA, et al. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid. 2014; 24:1121–1126. PMID: 24731094.

16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.

17. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015; 25:716–759. PMID: 25900731.

18. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010; 20:1341–1349. PMID: 21034228.

19. Sung TY, Jeon MJ, Lee YH, Lee YM, Kwon H, Yoon JH, et al. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2017; 102:793–800. PMID: 27809646.

20. Jeon MJ, Kim WG, Park WR, Han JM, Kim TY, Song DE, et al. Modified dynamic risk stratification for predicting recurrence using the response to initial therapy in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2013; 170:23–30. PMID: 24088549.

21. Krajewska J, Chmielik E, Jarząb B. Dynamic risk stratification in the follow-up of thyroid cancer: what is still to be discovered in 2017? Endocr Relat Cancer. 2017; 24:R387–R402. PMID: 28821573.

22. Schemper M. The relative importance of prognostic factors in studies of survival. Stat Med. 1993; 12:2377–2382. PMID: 8134740.

23. Lazar L, Lebenthal Y, Segal K, Steinmetz A, Strenov Y, Cohen M, et al. Pediatric thyroid cancer: postoperative classifications and response to initial therapy as prognostic factors. J Clin Endocrinol Metab. 2016; 101:1970–1979. PMID: 26930182.

24. Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011; 96:3615–3627. PMID: 21917876.
25. Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005; 365:2014–2023. PMID: 15950715.

26. Grigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002; 95:724–729. PMID: 12209714.

27. Alessandri AJ, Goddard KJ, Blair GK, Fryer CJ, Schultz KR. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med Pediatr Oncol. 2000; 35:41–46. PMID: 10881006.

28. Lee YA, Jung HW, Kim HY, Choi H, Kim HY, Hah JH, et al. Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J Clin Endocrinol Metab. 2015; 100:1619–1629. PMID: 25632969.

29. Golpanian S, Perez EA, Tashiro J, Lew JI, Sola JE, Hogan AR. Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int. 2016; 32:201–208. PMID: 26717936.

30. Zanella A, Scheffel RS, Pasa MW, Dora JM, Maia AL. Role of postoperative stimulated thyroglobulin as prognostic factor for differentiated thyroid cancer in children and adolescents. Thyroid. 2017; 27:787–792. PMID: 28292215.

Fig. 1
Disease-free survival curves according to American Thyroid Association risk stratification system (P = 0.041).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download