Abstract
Purpose
An oxaliplatin-based regimen is the most common adjuvant chemotherapy for patients with stage II/III colorectal cancer, but many patients experience dose reduction or early termination of chemotherapy due to side effects. We conducted this study to verify the range of reduction with oncologic safety.
Methods
Patients with stage II/III colorectal cancer who received adjuvant FOLFOX chemotherapy were enrolled in this study. The total amount of oxaliplatin administered per patient was calculated as a percentile based on 12 cycles of full-dose FOLFOX as a standard dose. The cutoff values showing significant differences in survival were calculated, and the clinicopathologic outcomes of patient groups classified by the value were compared.
Results
Among a total of 611 patients, there were 107 stage II patients, and 504 stage III patients. At 60% of the standard dose of oxaliplatin, the patients in the dose reduction group were older (62 years vs. 58 years, P = 0.003), had lower body mass index (BMI) (23.1 kg/m2 vs. 24.0 kg/m2, P = 0.005), and were more exposed to neoadjuvant treatment (18.0% vs. 9.1%, P = 0.003) in comparison to the standard group. At 60% of the standard dose, there were no significant differences in 5-year disease-free survival (DFS) and overall survival (OS) between the 2 groups (5-year DFS: 73.5% vs. 74.2%, P = 0.519; 5-year OS: 71.9% vs. 81.5%, P = 0.256, respectively).
Conclusion
Patients with old age, low BMI, and more frequent exposure to neoadjuvant treatment tended to show lower compliance with chemotherapy. More than 60% dose should be administered to patients with stage II/III colorectal cancer as adjuvant chemotherapy to achieve acceptable oncologic outcomes.
Adjuvant chemotherapy is one of the most important factors in the long-term oncologic outcomes of patients who undergo surgical resection for colorectal cancer (CRC) [12]. Previous randomized studies, including the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) study, have proven the superior therapeutic effect of oxaliplatin over 5-fluorouracil (FU) in adjuvant chemotherapy for CRC [3]. Currently, oxaliplatinbased FOLFOX chemotherapy is widely adapted as a standard adjuvant chemotherapy regimen for high-risk stages II and III CRC patients, resulting in improved patient survival [456].
The recommended dose of oxaliplatin is not frequently administered due to side effects such as bone marrow suppression or peripheral neuropathy [789]. Dose reduction may reduce the therapeutic effects of oxaliplatin, and it could lead to cancer recurrence or metastasis [10]. However, studies that directly evaluate the association between dose reduction of oxaliplatin and recurrence of CRC or survival are lacking. This study was designed to evaluate the high-frequency group for dose reduction of oxaliplatin and to define the acceptable range of dose reduction that will not influence oncologic outcome.
Patients who underwent surgery for CRC at Korea University Anam Hospital between September 2006 and December 2014, followed by adjuvant chemotherapy with FOLFOX6 regimen for stage II and III, were included in this study and reviewed retrospectively. The study was approved by the Institutional Review Board of Korea University Anam Hospital, and all patients provided informed consent. Based on the National Comprehensive Cancer Network guideline, stage II with risk factors (obstruction, perforation, inadequate lymph node sampling, or lymphovascular invasion) and stage III patients were recommended adjuvant FOLFOX6 chemotherapy. In our hospital, adjuvant chemotherapy was generally recommended for patients between the ages of 18 and 80. It was conducted considering the postoperative course and the patient's general condition.
The protocol with FOLFOX6 regimen at adjuvant setting used in our institution is as follows: 85 mg/m2 of oxaliplatin is administered via intravenous (IV) infusion at 150 mL per hour rate within 500 mL of 5% dextrose water at days 1–2, concurrent with 200 mg/m2 of leucovorin IV infusion, 400 mg/m2 of 5-FU in IV bolus next, then followed by 2 sets of 24-hour IV infusion of 600 mg/m2 of 5-FU solution. The resting periods follow for days 3–14. A maximum of 12 cycles is applied for every 2 weeks. The side effects of chemotherapy were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, and dose reduction was considered if the patient had any of the following side effects: hepatic impairment (higher than 3 times the normal range), renal impairment (creatinine clearance rate < 50 mL/min), febrile neutropenia (ANC < 1,000/mm3 plus fever of > 38.3℃ requiring IV antibiotics and hospitalization), thrombocytopenia (platelet count < 50 ×109/L), chemotherapy-induced peripheral neuropathy (CIPN) (grade > 2; moderate symptoms limiting instrumental activities of daily living such as meals, shopping, managing money), and Eastern Cooperative Oncology Group performance score ≥ 2. Because a specific questionnaire for CIPN was not used in this study, we considered patients requiring medication for neuropathy to be at grade 3 level of neurotoxicity. If a patient experiences significant adverse effects during the chemotherapy, dose reduction is considered for the next cycle and 75% to 80% of the original dose of oxaliplatin is administered as an initial dose reduction. If the adverse effects progress despite the initial dose reduction, a second dose reduction is considered and the administered dose of oxaliplatin drops to 50% to 60% of the original dose. If the progression of adverse symptoms still continues after the second dose reduction, chemotherapy is terminated early.
The total amount of oxaliplatin administered was calculated as a percentile, reflecting the degree of reduction and cycle. Full administration of a standard dose of oxaliplatin for 12 cycles was set as the reference dosage. The dose-reduction group and standard dose group were divided according to each 5% dose reduction, and then overall survival (OS) and disease-free survival (DFS) of the 2 groups were compared at every point. The cutoff value was defined at the lowest dose of oxaliplatin administered without significant differences in either OS or DFS. Patient demographics, tumor characteristics, operative and postoperative outcomes were compared between groups.
Descriptive results are presented as mean with standard deviation or median with interquartile range (Q1–Q3) for continuous outcomes and as frequency and percentage for categorical outcomes. Student t-test was used to compare continuous variables, and the chi-square test or Fisher exact test was applied for categorical variables. Five-year OS and DFS were analyzed using the Kaplan-Meier method, and comparison of survival between groups was performed by log-rank test. Linear regression test was used to analyze the change in OS and DFS in regards to dosage, and univariate and multivariate coxregression was analyzed to identify the risk factors for survival. Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). A P-value <0.05 was considered statistically significant.
Among 2,761 patients who underwent surgery during the study period, 1,400 received adjuvant chemotherapy as stage II with high risk and stage III. A total of 611 patients who received FOLFOX6 regimen as adjuvant chemotherapy were enrolled in this study. There were 365 male (59.7%) and 246 female patients (40.3%). The mean age was 59.2 years and the mean body mass index (BMI) was 24.8 kg/m2. Among the patients, 391 were diagnosed with colon cancer, 205 had rectal cancer, and 15 patients had cancer lesions in both colon and rectum. The mean level of preoperative CEA was 5.9 ng/mL. There were 68 patients who received neoadjuvant treatment, including concurrent chemoradiotherapy in most, and emergency operation was performed in 16 patients. The mean operation time was 223.4 minutes and mean estimated blood loss was 127.5 mL. The mean postoperative hospital stay was 11.5 days. There were 107 stage II patients and 504 stage III patients, and the median follow-up duration was 69 months.
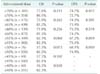
Only 84 patients (13.8%) completed the adjuvant chemotherapy with full-dose oxaliplatin, and the mean administered dose of oxaliplatin was 70% of the standard dosage (Table 1). There was no significant difference for patients who underwent adjuvant chemotherapy 8 weeks postoperatively due to reasons like complication between the groups (5.8% vs. 2.5%, P = 0.061). The median day for administration was 21 days in the reduction group and 17 days in the standard group (P = 0.108). The comparisons of OS and DFS in the reduction group and the standard groups, which were divided according to each 5% dose reduction, are shown in Table 2. The survival rates were significantly decreased in direct proportion to the dose reduction of oxaliplatin (P = 0.001, R2 = 0.945 for OS; P = 0.043, R2 = 0.889 for DFS). With 55% of the standard dose of oxaliplatin, the 5-year DFS was significantly different with 67.2% in the reduction group and 75.5% in the standard group (P = 0.039), but there was no significant difference in 5-year OS with 62.3% in the reduction group and 82.3% in the standard group (P = 0.068). With 40% of the standard dose, there was a significant difference in 5-year OS between the two groups (36.5% vs. 82.3%, P = 0.030).
The cutoff value was set at 60% of the standard dose as the lowest dose of oxaliplatin required to maintain the oncologic outcome. The results comparing the reduction group and the standard group, which are divided at the 60% dose, are shown in Tables 3, 4, 5. The mean amount of oxaliplatin administered was 80.0% in the standard group and 70.5% in the dose reduction group. The reduction group included older patients (62 years vs. 58 years, P = 0.003), with lower BMI (23.1 kg/m2 vs. 24.0 kg/m2, P = 0.005), and more frequently exposed to neoadjuvant treatment (18.0% vs. 9.1%, P = 0.003) than those of the standard group (Table 3). In terms of chemotherapyrelated toxicity, patients with neutropenic event including febrile neutropenia did not differ between the 2 groups, but patients in the standard group showed significant higher utilization of medication than in the reduction group (14 [10.1%] vs. 139 [29.4%], P < 0.001) (Table 5). There was no FOLFOX chemotherapy-related mortality reported in this study.
The median follow-up duration was 31.1 months in the dose reduction group and 34.2 months in the standard group The survival differences were not significant in both the 5-year DFS and 5-year OS between the 2 groups at 60% of the standard oxaliplatin dose (5-year DFS, 73.5% vs. 74.2%, P = 0.519; 5-year OS, 71.9% vs. 81.5%, P = 0.256, respectively) (Fig. 1). Based on the subgroup analysis for survivals by N stage, most OS and DFS were not significantly different between the 2 groups, but only OS in the N1 subgroup showed a significance (82.7% vs. 90.2%, P = 0.043). On univariate analysis for OS, operative methods had a significant influence on OS (laparoscopy: P = 0.006, OR 0.236; robot: P = 0.002, OR 0.137). In addition, high CEA, long operation time, long postoperative days, tumor size, and higher number of positive lymph nodes were negatively associated with OS (CEA: P = 0.021, OR 1.012; operation time: P < 0.001, OR 1.004; postoperative days: P < 0.001, OR 1.054; tumor size P = 0.040, OR 1.026; positive lymph nodes: P < 0.001, OR 1.058). Multivariate analysis showed that operation time and number of positive lymph nodes were independently significant predictors of OS (operation time: P = 0.027, OR 1.003; positive lymph nodes: P < 0.001, OR 1.076) (Table 6).
Our study showed that only 13.7% of the patients with stages II and III CRC completed adjuvant FOLFOX6 chemotherapy at full dose, and survival significantly decreased in proportion to oxaliplatin dose reduction. More than 60% of the standard dose is necessary to maintain oncological outcomes similar to those of the standard dose, although more medications for CIPN were significantly required than in the reduction group receiving oxaliplatin at 60% or less. Patients in the dose reduction group tended to be older and to have lower BMI and frequent exposure to neoadjuvant treatment according to a dose cutoff value at 60%.
The FOLFOX regimen has become the standard chemotherapy in CRC treatment [245]. Previous large studies, including the MOSAIC study, have proven that oxaliplatin combined with 5-fluorouracil chemotherapy has oncologic superiority compared to conventional 5-fluorouracil single regimen as an adjuvant chemotherapy for the treatment of CRC [1361112]. Oxaliplatin is a platinum-based drug that has cytotoxic effects due to interference with DNA and inhibition of DNA synthesis, which results in apoptosis in cancer cells [1314]. However, oxaliplatin also alters the function of the voltagegated sodium channels involved with calcium in neuron cells, leading to axon degeneration. In addition, the platinum compound accumulates in the dorsal root ganglia cells, which causes atrophy of neuron cells [71516]. Peripheral neuropathy is one of the most common adverse effects of oxaliplatin, with more than 15% of patients developing grade 3 or 4 neuropathy with a cumulative dose of about 800 mg/m2 [917]. Although irreversible neuropathy is known to occur in about 5%, the actual patient discomfort experienced during the treatment is frequently severe, and greatly influences the compliance with chemotherapy and its dose reduction [7915].
In the Adjuvant Colon Cancer with Eloxatin study, the majority of patients in the registry were treated with only 6 cycles of an average FOLFOX regimen, and there was a significant difference between the recommended dose and the actual dose administered to patients [18]. The efficacy for the prevention of CRC recurrence is questionable with the reduced oxaliplatin dose [1920], but the studies are lacking. Some studies are currently underway such as the MIDAS trial (protocol No. CRAD001CKR13T).
Our study showed that the survival rate is significantly negatively associated with the dose reduction of oxaliplatin. Similarly, Tsai et al. [21] showed that FOLFOX chemotherapy should be administered as at least 8 cycles to maintain the benefit for OS, and at least 7 cycles for DFS. In addition, Maindrault-Goebel et al. [22] showed that high-dose oxaliplatin treatment has significant improvement in the rate of disease response and progression-free survival compared to low-dose intensity. Because DFS showed significant differences at 55% of the standard dose, we set the cutoff value at 60% of standard dose in our study so that survival was not influenced. The dose reduction group had more older patients with lower BMI, and they were suspected to have poor general condition with lower tolerability of FOLFOX chemotherapy. Furthermore, more patients in the dose reduction group were exposed to neoadjuvant treatment, and they may have received less adjuvant therapy due to the fatigability with prolonged overall treatment. In addition, the dose reduction group tended to have more risk factors for survival. Nevertheless, it is very informative that oncologic outcomes in the patients receiving 60% of standard dose of oxaliplatin were comparable to those of the patients in the standard group.
This study has several limitations. First, patients in both groups were heterogeneous, and there was a considerable difference in the number of patients in the 2 groups. Thus, our results should be carefully interpreted. Second, there was no comparison with the group of patients who received no chemotherapy, and we thus should not conclude that the administration of an oxaliplatin dose below 60% does not have survival benefits. If the dose reduction group shows superior oncologic outcomes to the no-chemotherapy group, chemotherapy administration would be justified despite the side effects. On the other hand, even if the oncologic results of the dose reduction group were comparable or inferior to those in no-chemotherapy group, chemotherapy should be tried because it is never clear whether patients will require dose reduction throughout the treatment period. Therefore, a comparison between reduced-dose and no-chemotherapy groups is necessary to evaluate the value of reduced dose administration. Furthermore, the no-chemotherapy group may include many patients with a poor general condition, making it impossible to administer chemotherapy, and careful interpretation will be necessary. Finally, medical records in terms of side effects during the chemotherapy period, especially grading scale of toxicity severity, were lacking due to the nature of the retrospective study. Also, evaluation of the relationship between side effects and dose reduction could not be performed quantitatively in our study.
In conclusion, older patients with lower BMI and more exposure to neoadjuvant treatment showed low compliance with chemotherapy in our study. More than 60% of the standard dose of oxaliplatin should be administrated to patients with stage II/III CRC as adjuvant chemotherapy to achieve comparable oncologic results to those of the standard dose group.
Figures and Tables
Fig. 1
Five-year disease-free survival (A) and overall survival (B) for the dose reduction group and the standard group at the reduced dose of oxaliplatin (60%).
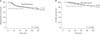
Table 2
Comparison of survival between the dose reduction group and the standard group according to the administered dose of oxaliplatin
Notes
References
1. Sharif S, O'Connell MJ, Yothers G, Lopa S, Wolmark N. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon ancer. Cancer Invest. 2008; 26:956–963.
2. Chau I, Cunningham D. Chemotherapy in colorectal cancer: new options and new challenges. Br Med Bull. 2002; 64:159–180.

3. Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350:2343–2351.

4. Andre T, Afchain P, Barrier A, Blanchard P, Larsen AK, Tournigand C, et al. Current status of adjuvant therapy for colon cancer. Gastrointest Cancer Res. 2007; 1:90–97.
5. Rothenberg ML. Efficacy of oxaliplatin in the treatment of colorectal cancer. Oncology (Williston Park). 2000; 14:12 Suppl 11. 9–14.
6. Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009; 27:3109–3116.
7. Argyriou AA. Updates on oxaliplatin-induced peripheral neurotoxicity (OXAIPN). Toxics. 2015; 3:187–197.
8. Kuebler JP, Colangelo L, O'Connell MJ, Smith RE, Yothers G, Begovic M, et al. Severe enteropathy among patients with stage II/III colon cancer treated on a randomized trial of bolus 5- fluorouracil/leucovorin plus or minus oxaliplatin: a prospective analysis. Cancer. 2007; 110:1945–1950.
9. Grothey A. Clinical management of oxaliplatin- associated neurotoxicity. Clin Colorectal Cancer. 2005; 5:Suppl 1. S38–S46.
10. Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, Andre T, Bennamoun M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009; 27:5727–5733.

11. Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007; 25:2198–2204.

12. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947.

14. Alcindor T, Beauger N. Oxaliplatin: a review in the era of molecularly targeted therapy. Curr Oncol. 2011; 18:18–25.

15. Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008; 34:368–377.

16. Pasetto LM, D'Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006; 59:159–168.

17. Argyriou AA, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T, Gerolymos MK, et al. Incidence and characteristics of peripheral neuropathy during oxaliplatin-based chemotherapy for metastatic colon cancer. Acta Oncol. 2007; 46:1131–1137.

18. Park YS, Ji J, Zalcberg JR, El-Serafi M, Buzaid A, Ghosn M. Oxaliplatin/5-fluorouracil-based adjuvant chemotherapy as a standard of care for colon cancer in clinical practice: outcomes of the ACCElox registry. Asia Pac J Clin Oncol. 2015; 11:334–342.

19. Aspinall SL, Good CB, Zhao X, Cunningham FE, Heron BB, Geraci M, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015; 15:62.

20. Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006; 24:394–400.

21. Tsai YJ, Lin JK, Chen WS, Jiang JK, Teng HW, Yen CC, et al. Adjuvant FOLFOX treatment for stage III colon cancer: how many cycles are enough. Springerplus. 2016; 5:1318.

22. Maindrault-Goebel F, de Gramont A, Louvet C, André T, Carola E, Gilles V, et al. Evaluation of oxaliplatin dose intensity in bimonthly leucovorin and 48-hour 5-fluorouracil continuous infusion regimens (FOLFOX) in pretreated metastatic colorectal cancer. Oncology Multidisciplinary Research Group (GERCOR). Ann Oncol. 2000; 11:1477–1483.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download