Abstract
Purpose
The clinical behavior of T2 gallbladder cancer varies among patients. The aims of this study were to identify prognostic factors for survival and recurrence, and to determine the optimal surgical strategy for T2 gallbladder cancer.
Methods
We conducted a retrospective analysis of 78 patients with T2 gallbladder cancer who underwent surgical resection for gallbladder cancer.
Results
Twenty-eight patients (35.9%) underwent simple cholecystectomy and 50 (64.1%) underwent extended cholecystectomy. Among 56 patients without LN metastasis (n = 20) or unknown LN status (no LN dissection, n = 36), the 5-year disease-free survival rates were 81.6%, and 69.8% (P = 0.080). In an analysis according to tumor location, patients with tumors located on the hepatic side (n = 36) had a higher recurrence rate than patients with tumors located on the peritoneal side only (n = 35) (P = 0.043). On multivariate analysis, R1 resection and lymph node metastasis were significant, independent prognostic factors for poor disease-free and overall survival.
Gallbladder cancer is the most frequent malignant neoplasm of the biliary tract. Unfortunately, the prognosis of gallbladder cancer, except for early-stage cancer, is very poor. The depth of tumor invasion and lymph node (LN) metastasis were reported to be the most important prognostic factors for gallbladder cancer [1]. T1 carcinoma confined to the lamina propria (T1a) or muscle layer (T1b) had favorable 5-year survival rates of 72%–100% in earlier studies [2345] and simple cholecystectomy is considered to be an adequate surgical approach for T1 gallbladder cancer [6]. Meanwhile, the prognosis of T3 and T4 gallbladder cancer with serosal involvement and invasion into adjacent organs or main vessels is still very dismal with 5-year survival rates of <20% [27]. As compared with T1, T3, and T4 gallbladder cancer, the prognosis of T2 gallbladder cancer is very heterogeneous and is difficult to predict. Moreover, T2 was the most common T stage of gallbladder cancer in several studies [578]. Therefore, further improvements in the overall survival rate of gallbladder cancer could be achieved by improving the survival of patients with T2 gallbladder cancer.
There is a hope for long-term survival only in the case of complete resection and if there are no residual tumor cells. The recently published National Comprehensive Cancer Network guidelines and American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition) recommend extended cholecystectomy including both LN dissection and hepatic resection. However, the extent of extended cholecystectomy has not been defined and the adequate surgical resection approach for T2 gallbladder cancer is still debated.
The aims of this retrospective study were to identify prognostic factors for survival and recurrence, and to determine an adequate surgical strategy for T2 gallbladder cancer, focusing on the role of hepatic resection.
This was a retrospective study and approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (approval number: 2016-01-040-001) with agreement exemption for informed consent.
Between December 2000 and August 2012, 165 patients with gallbladder cancer underwent complete resection at a tertiary hospital in Daegu, Korea. Of these, 78 patients (47.2%) had pT2 gallbladder cancer. R1 resection was performed in 4 patients and R0 resection in 74 patients. The following preoperative demographic and clinical characteristics were retrospectively obtained from the patients' medical records: age, sex, types of operative procedure, tumor markers, operation time, need for transfusion, postoperative complications, hospital stay, and mode of recurrence. The location of the tumor was defined based on preoperative radiologic images, mostly CT scan. Hepatic side gallbladder cancer was defined when tumor was located within gallbladder bed attached to the liver. Tumor located only the free serosa side of gallbladder was classified as peritoneal side gallbladder cancer. If extent of tumor included both peritoneal and hepatic side, it was categorized as hepatic side cancer.
To avoid confusion regarding the surgical terms, we used the following definitions. Radical cholecystectomy was defined as all procedures beyond simple cholecystectomy. Extended cholecystectomy was defined as simple cholecystectomy with LN dissection and hepatic resection of S4b and S5 or gallbladder bed resection with a 2- to 3-cm margin. Laparoscopic resection was performed if T1 cancer was suspected based on the patient's preoperative radiological findings. After cholecystectomy, the specimen was sent for frozen sectioning. If the tumor invaded perimuscular connective tissue, the procedure was converted to open surgery for extended resection. If the depth of invasion was uncertain, additional procedures or closure without extended resection were selected on a case-by-case basis. For patients who initially underwent simple cholecystectomy, additional extended resection was performed after confirming the patient's histopathological findings.
We intentionally performed extended cholecystectomy, at least, in patients with T2 gallbladder cancer. However, if the patient refused to undergo additional extended resection by open laparotomy or had severe comorbidities, even if extended resection was necessary on the basis of the pathologic findings after simple cholecystectomy, the patient was very carefully followed up without additional procedures.
Bile duct resection was performed if the tumor was located at the infundibulum or near the cystic duct. Partial hepatic resection (wedge resection or S4b and S5 resection) was done if the tumor was located in the liver bed. Typically, regional LN dissection (Nos. 8, 12, 13) was performed and aortocaval LN dissection was considered for selected patients who had suspicious finding in preoperative radiologic studies.
The following microscopic characteristics were evaluated: differentiation, the presence of lymphovascular and perineural invasion, LN metastasis, and margin status. R0 resection was defined as margin-negative resection in the pathologic report and the absence of grossly residual tumors, LN enlargement, and distant metastasis in the operative and radiologic findings. R1 resection was defined as the presence of residual tumor cells under microscopy. R2 resection was defined as the presence of a macroscopic residual tumor, but there were no R2 cases among the 78 patients with T2 gallbladder cancer. Cancer stage was evaluated according to the AJCC Cancer Staging Manual (7th edition).
The surgical outcomes and prognostic factors were retrospectively evaluated based on these factors. Statistical analysis was performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). The survival time was calculated from the date of surgery to the date of death or the last follow-up. Disease-free survival was calculated from the date of surgery to the date of recurrence. Survival was analyzed using the Kaplan-Meier method, and variables were compared using the log-rank test. Multivariable regression analysis was performed using the Cox proportional hazards model to identify independent prognostic factors for survival. P-values of <0.05 were considered statistically significant.
The demographic characteristics and perioperative data, including the types of surgical procedures, are shown in Table 1. Among 78 patients with T2 stage gallbladder, 32 (28.2%) were men and 46 (71.8%) were women, and the mean age was 68.3 years (range, 44–87 years). Twenty-eight patients (35.9%) underwent simple cholecystectomy and 50 patients (64.1%) underwent extended cholecystectomy. One patient underwent pancreaticoduodenectomy because the tumor cells had spread into the distal bile duct from cystic duct cancer. Twenty-four patients (30.8%) underwent laparoscopic surgery and 54 patients (69.2%) underwent open surgery. Nine surgery-related complications (11.5%) occurred. Bile leakage was found in 3 patients, delayed gastric emptying in 3 patients, and bleeding in 1 patient. There were no intraoperative deaths.
The clinicopathological outcomes are shown in Table 2. The mean tumor diameter was 28.4 mm (range, 3–90 mm). The tumor was located on the peritoneal side in 35 patients, on the hepatic side in 5 patients, and involved both sides in 31 patients. The tumor was located in the fundus and/or body of the gallbladder in 58 patients (74.4%), the infundibulum to neck in 12 patients (15.3%), the cystic duct in 3 patients (3.8%), and the entire gallbladder wall in 5 patients (6.4%). The tumor was classified as well differentiated in 14 patients (17.9%), moderately differentiated in 49 patients (62.8%), and poorly differentiated in 8 patients (10.2%). R0 resection was achieved in 74 patients and R1 resection was achieved in 4 patients.
During the follow-up period of 45.4 months, 22 patients (28.2%) experienced disease recurrence. The disease-free survival rates among the 78 patients who underwent surgical resection were 64.9% at 3 years and 62.6% at 5 years. The sites of recurrence or distant metastasis were regional LN metastasis in 13 patients, distant LN metastasis in 1 patient, and lung metastases in 2 patients. Hepatic metastases were found in 6 patients (27.2%); liver resection was performed in 3 and not in the other 3. Eighteen patients died because of disease recurrence (Fig. 1). The overall survival rates of the 78 patients were 70.9% at 3 years and 64.6% at 5 years.
The 5-year disease-free survival rates of patients with and without LN metastasis were 81.8% and 34.7%, respectively (P = 0.020) (Fig. 1A). Patients who underwent R0 resection (n = 74) showed significant better disease-free survival than patients who underwent R1 resection (n = 4) (P = 0.003) (Fig. 1B).
The disease-free survival rate was similar in patients who underwent simple cholecystectomy and radical cholecystectomy, with 5-year survival rates of 66.5% and 59.5%, respectively (P = 0.838) (Fig. 2A). Among 56 patients without LN metastasis (N0 after LN dissection, n = 20) or unknown LN status (Nx due to no LN dissection, n = 36), the 5-year disease-free survival rates were 81.6%, and 69.8% (P = 0.080) in patients who underwent LN dissection or not, respectively (Fig. 2B). Disease recurrence was found in 2 patients (10.0%) with LN dissection and in 11 patients (30.5%) without LN dissection.
In a subgroup analysis according to tumor location, patients with tumors located on the hepatic side (n = 36, hepatic side + both hepatic and peritoneal side) had a higherer recurrence rate than patients with tumors located on the peritoneal side only (n = 35) (P = 0.043) (Fig. 3A). However, in patients with tumors on the hepatic side, liver resection did not affect long-term survival (P = 0.846) (Fig. 3B).
Disease-free survival was not affected by the tumor location in the gallbladder (i.e., fundus, body, infundibulum, and cystic duct).
The results of the univariate analyses of the clinicopathological characteristics of pT2 gallbladder cancer are shown in Table 3. Univariate analyses showed that R1 resection, absence of LN metastasis (P = 0.020), lymphovascular invasion, and tumor stage were significant prognostic factors for poor disease-free and overall survival. In addition, the multivariable analyses showed that R1 resection and LN metastasis were significant and independent prognostic factors for poor disease-free and overall survival (Table 3).
Because of its vague symptoms and aggressive clinical behavior, most gallbladder cancer is at an advanced stage at diagnosis. Therefore, the prognosis of gallbladder cancer is poor, and long-term survival is only realistic if it is detected early and complete surgical resection is performed [9]. The 5-year survival rate of T2 gallbladder cancer varied considerably in earlier studies, ranging from 29.3% to 78.3% [341011]. In our study, the 5-year survival rate was 62.6%, consistent with that of earlier reports.
Several clinicopathological factors were reported to affect the prognosis of gallbladder cancer, including LN metastasis, lymphatic invasion, vascular invasion, perineural invasion, differentiation grade, tumor-node-metastasis stage, and residual tumor status [4101213]. The results of the univariate analyses in our study yielded similar prognostic factors. However, only R0 resection and LN metastasis were significant prognostic factors for overall survival in the multivariable analysis.
Extended cholecystectomy, including LN dissection and hepatic resection, was reported as a significant surgery-related prognostic factor. In patients who undergo simple cholecystectomy alone, the postoperative survival is dismal, with 5-year survival rates of 17%–40% reported [51415]. Shirai et al. [5] conducted a retrospective analysis of 48 patients and found that radical resection of pT2 gallbladder cancer improved the 5-year survival rate from 40% to 90%. On the basis of these studies, the AJCC Cancer Staging Manual, 7th edition, and the recently published guidelines of the Korean Association of HBP Surgery recommend extended cholecystectomy with regional LN dissection and en bloc hepatic resection in patients with T2 gallbladder cancer. However, extended cholecystectomy was performed in just 13.4%–45.8% of patients with T2 gallbladder cancer in clinical practice [101617]. Despite this inconsistency, some studies have reported a favorable survival rate after simple cholecystectomy [3], and we found a high 5-year overall survival rate of 66.5% in patients who underwent simple cholecystectomy. Although survival was not significantly different (P = 0.080) between N0 and Nx patients (n = 56), patients who underwent LN dissection showed favorable survival relative to patients who did not undergo LN dissection (Fig. 2B). The rate of LN metastasis in T2 gallbladder cancer was reported to be as high as 63.4% [14182021], and considering LN metastasis is an independent significant prognostic factor, LN dissection should be performed to achieve R0 resection if there are no specific contraindications to this procedure. The present study included 28 patients (35.9%) who underwent simple cholecystectomy, and most of them were treated before the policy for routine LN dissection was established. Therefore, our results show that LN dissection is necessary in patients with T2 gallbladder cancer.
Some recent studies have suggested that tumor location on the hepatic side of the gallbladder is a significant prognostic factor for poor survival [2223]. Microscopic liver metastasis is frequently detected in patients with T2 gallbladder cancer, and residual cancer cells were more frequently detected in the gallbladder bed adjacent to the liver parenchyma after simple cholecystectomy in patients with tumors on the hepatic side [2224]. Similar to our study, Shindoh et al. [22] reported that tumors located on the hepatic were associated with poor prognosis in patients with T2 cancer, but not in patients with T1 or T3 cancer. The need for hepatic resection of T2 gallbladder cancer is still controversial [21]. Hepatic S4b and S5 resection or gallbladder bed resection with a 2- to 3-cm margin have been performed based on the fact that most of the cholecystic vein drains through segments 4 and 5 [25]. However, invasion of gallbladder cancer into the gallbladder vein was reported in just 0%–10% of all cases [2627]. Furthermore, the most common event is direct invasion of the tumor into adjacent liver segments 4 and 5, which is classified as pT3. There was no evidence of intrahepatic intravascular invasion in cases of gross liver invasion of gallbladder cancer in an early study of the modes of spread, and vascular invasion is a relatively rare mode of spread of gallbladder cancer [26]. Therefore, tumor location on the hepatic side is clearly associated with poor prognosis. Nevertheless, the benefit of hepatic resection of tumors located on the hepatic side remains unclear. Our study showed that liver resection in patients located on the hepatic side did not affect long-term survival, similar to the study by Shindoh et al. [22]. However, Lee et al. [23] reported that liver resection had a beneficial effect on long-term survival in patients with T2 cancer located on the hepatic side but not in patients with tumors located on the peritoneal side.
In conclusion, R0 resection and LN dissection are an appropriate curative surgical strategy in patients with T2 gallbladder cancer. Although tumors located on the hepatic side show worse prognosis than tumors located on the peritoneal side, the need for hepatic resection should be evaluated in additional studies.
Figures and Tables
Fig. 1
(A) The 5-year disease-free survival rates of patients with (N1) and without (N0) lymph node metastasis were 81.8% and 34.7%, respectively (P = 0.020). (B) Patients who underwent R0 resection (n = 74) showed significant better disease-free survival than patients who underwent R1 resection (n = 4) (P = 0.003).
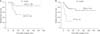
Fig. 2
(A) The disease-free survival rate was similar in patients who underwent simple cholecystectomy (simple) and radical cholecystectomy (radical), with 5-year survival rates of 66.5% and 59.5%, respectively (P = 0.838). (B) Between 56 patients without lymph node (LN) metastasis (N0 after lymph node dissection, n = 20) or unknown LN status (NX due to no lymph node dissection, n = 36), the 5-year disease-free survival rates were 81.6%, and 69.8% (P = 0.080) in patients who underwent LN dissection or not, respectively.
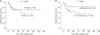
Fig. 3
Analysis according to the tumor location. (A) Patients with tumors located on the hepatic side (n = 36, hepatic side + both hepatic and peritoneal side) had a higherer recurrence rate than patients with tumors located on the peritoneal side only (n = 35) (P = 0.043). (B) In patients with tumors on the hepatic side, liver resection did not affect long-term survival (P = 0.846).
Table 1
Demographics and perioperative characteristics of patients with T2 gallbladder cancer (n = 78)

References
1. Yamaguchi K, Chijiiwa K, Saiki S, Nishihara K, Takashima M, Kawakami K, et al. Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg. 1997; 84:200–204.

2. Ogura Y, Mizumoto R, Isaji S, Kusuda T, Matsuda S, Tabata M. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991; 15:337–343.

3. Kang CM, Lee WJ, Choi GH, Kim JY, Kim KS, Choi JS, et al. Does “clinical” R0 have validity in the choice of simple cholecystectomy for gallbladder carcinoma? J Gastrointest Surg. 2007; 11:1309–1316.

4. Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extrahepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol. 2008; 97:498–502.

5. Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992; 215:326–331.
6. Lee SE, Jang JY, Lim CS, Kang MJ, Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011; 17:174–180.

7. Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009; 16:1–7.

8. Chan SY, Poon RT, Lo CM, Ng KK, Fan ST. Management of carcinoma of the gallbladder: a single-institution experience in 16 years. J Surg Oncol. 2008; 97:156–164.

9. Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006; 93:670–681.

10. Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg. 2010; 14:668–678.

11. Suzuki S, Yokoi Y, Kurachi K, Inaba K, Ota S, Azuma M, et al. Appraisal of surgical treatment for pT2 gallbladder carcinomas. World J Surg. 2004; 28:160–165.

12. Chijiiwa K, Noshiro H, Nakano K, Okido M, Sugitani A, Yamaguchi K, et al. Role of surgery for gallbladder carcinoma with special reference to lymph node metastasis and stage using western and Japanese classification systems. World J Surg. 2000; 24:1271–1276.

13. Wakai T, Shirai Y, Yokoyama N, Ajioka Y, Watanabe H, Hatakeyama K. Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol. 2003; 10:447–454.

14. Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001; 192:600–607.

15. Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998; 175:118–122.
16. Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg. 2010; 14:1578–1591.
17. Kim DH, Kim SH, Choi GH, Kang CM, Kim KS, Choi JS, et al. Role of cholecystectomy and lymph node dissection in patients with T2 gallbladder cancer. World J Surg. 2013; 37:2635–2640.

18. Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997; 79:892–899.

19. Isambert M, Leux C, Métairie S, Paineau J. Incidentally-discovered gallbladder cancer: When, why and which reoperation? J Visc Surg. 2011; 148:e77–e84.

20. Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg. 2008; 247:104–108.
21. Yokomizo H, Yamane T, Hirata T, Hifumi M, Kawaguchi T, Fukuda S. Surgical treatment of pT2 gallbladder carcinoma: a reevaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the long-term outcome. Ann Surg Oncol. 2007; 14:1366–1373.

22. Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg. 2015; 261:733–739.
23. Lee H, Choi DW, Park JY, Youn S, Kwon W, Heo JS, et al. Surgical strategy for T2 gallbladder cancer according to tumor location. Ann Surg Oncol. 2015; 22:2779–2786.

24. Endo I, Shimada H, Takimoto A, Fujii Y, Miura Y, Sugita M, et al. Microscopic liver metastasis: prognostic factor for patients with pT2 gallbladder carcinoma. World J Surg. 2004; 28:692–696.

25. Yoshimitsu K, Honda H, Kaneko K, Kuroiwa T, Irie H, Chijiiwa K, et al. Anatomy and clinical importance of cholecystic venous drainage: helical CT observations during injection of contrast medium into the cholecystic artery. AJR Am J Roentgenol. 1997; 169:505–510.

26. Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg. 1962; 156:114–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download