Abstract
Purpose
Gastric emptying may influence the quality of life of patients who undergo distal gastrectomy. Little is known, however, about gastric emptying after distal gastrectomy. The aim of our study was to investigate gastric emptying patterns after distal gastrectomy.
Methods
This gastric-emptying study investigated patients who underwent distal gastrectomy in the 6 months or more before May 2008 to July 2013 at Chungbuk National University Hospital with a study sample of 205 patients. We analyzed patterns of gastric emptying.
Results
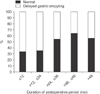
Delayed gastric emptying was found in 109 of the 205 patients (53.2%). Food stasis was more frequent in a group with delayed gastric emptying. In multivariate analysis, risk factors for gastroparesis were laparoscopic operation (hazard ratio [HR], 2.731; P = 0.008) and duration of less than 24 months after distal gastrectomy (HR, 2.795; P = 0.001). Delayed gastric emptying tended to decrease with duration of the postoperative period.
Gastric cancer is the second most common cancer in Korea, and the third leading cause of cancer deaths [1]. The survival rate of gastric cancer has improved due to early diagnosis, advances in multimodality management and decreased mortality, such that the number of survivors has increased [2]. Thus, there have been recent advances for improving quality of life (QoL) after gastrectomy in the treatment of gastric cancer such as endoscopic mucosal resection, endoscopic submucosal dissection, sentinel lymph node navigation surgery and pylorus-preservation gastrectomy. Nonetheless, the principal treatment for gastric cancer is still extensive dissection of the lymph node, with primary tumor resection [34]. Patients who undergo distal gastrectomy often have postgastrectomy syndromes such as delayed gastric emptying, dumping syndrome, reduced food intake and reflux esophagitis, which reduce QoL after gastrectomy [5]. Patients who undergo gastrectomy have poorer QoL than before surgery. In the immediate postoperative period, the majority of patients have worse QoL. Most patients recover their QoL, but onethird experience a maintained worse QoL after surgery [6]. Poorer QoL than before distal gastrectomy may be related to postgastrectomy syndrome such as rapid or delayed gastric emptying of the remnant stomach. Recovery of QoL after distal gastrectomy is assumed to be related to functional changes of the remnant stomach. After distal gastrectomy, the remnant stomach may have a critical role in postgastrectomy syndrome. However, the function of the remnant stomach after distal gastrectomy is not well understood, and little is known about gastric emptying patterns after distal gastrectomy. The aim of our study was to evaluate risk factors for delayed gastric emptying and to investigate chronological functional changes of the remnant stomach after distal gastrectomy.
Two hundred five patients who were tested for gastric emptying between April 2013 and July 2014 were selected from a population of 465 patients who were pathologically diagnosed with gastric cancer and underwent distal gastrectomy from August 2008 to July 2013 at Chungbuk National University Hospital. We retrospectively analyzed risk factors for gastroparesis and chronological changes according to postoperative duration after gastrectomy. All patients underwent radical gastrectomy for primary tumor and D2 lymph node dissection for cases of AGC and D2 or D+ lymph node dissection for cases of early gastric cancer (EGC) by a single surgeon. Truncal vagotomy was routinely performed. BillrothI reconstruction was conducted using a 28mm diameter circular stapler (Autosuture, Covidien, Norwalk, CT, USA). Handsewn anastomosis was performed for BillrothII with Braun anastomosis, and endtoside gastrojejunostomy was made approximately 30 cm distal to the ligament of Treitz via the anterocolic and isoperistaltic pathway. Braun anastomosis was performed about 40 to 50 cm distal to the gastrojejunostomy. In the laparoscopic surgery group, all anastomoses were performed extracorporeally.
We performed gastric emptying study the patients who visited the outpatients department from January 2012 to September 2014. The enrolled patients did not receive chemotherapy, did not recur gastric cancer and did not occur metachronous primary tumor in the other organ. It was carried out regardlness of the manifestration of symptoms. Gastric emptying was studied in patients with distal gastrectomy after 6 months postoperative. Studies used 99mtechnetium sulfur colloidradiolabeled boiled eggs. All patients who underwent gastric-emptying studies fasted at least 8 hours before study initiation. Radiolabeled eggs were ingested within 10 minutes before the test. Normal values for gastric-emptying time were defined as a halftime emptying range of 27–72 minutes. A halftime for emptying of more than 72 minutes was defined as delayed gastric emptying. Gastric emptying was divided into 3 grades according to time. Grade 1 was defined as gastric emptying time <72 minutes, grade 2 as ≥72 to <144 minutes and grade 3 as >144 minutes [8]. Gastroscopy was performed at the same time as the gastric-emptying studies. Reflux esophagitis was assessed based on endoscopic findings, and gastric stasis was defined as higher than grade 1 in the residue system, as reported by Kubo et al. [7]. We retrospectively analyzed risk factors for delayed gastric emptying and chronological changes in gastroparesis after distal gastrectomy. All statistical analyses used IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Chisquare tests were used to analyze clinicopathogical findings, and Cox proportional hazards models were used for multivariate analysis. A P-value < 0.05 was considered statistically significant.
Among the 205 patients, 129 patients were men and 76 were women with a mean age of 59.6 ± 10.4 years. Mean time between distal gastrectomy and the gastric-emptying study period was 26.7 ± 17.2 months. Prior to 2011, 55 patients (57.3%) underwent laparoscopic surgery, and after 2012, 73 patients (67.0%) underwent laparoscopic surgery. There was no statistical difference of ratio of laparoscopic surgery between the 2 periods when divided by 2011 (P = 0.153). In univariate analysis, age, sex, type of reconstruction, body mass index (BMI), tumor stage, location of tumor, and diabetes mellitus (DM) were not risk factors for gastroparesis. However, 79 patients (61.7%) who underwent laparoscopic gastrectomy showed gastroparesis out of 128 patients (P = 0.002). The 64 patients (65.3%) with less than 24 months between surgery and the gastric-emptying study showed more frequent gastroparesis (P = 0.001). Patients with gastroparesis were more frequently observed to have gastric stasis (P < 0.001). Reflux esophagitis was frequently observed in the gastroparesis group, but this observation was not significant (P = 0.056) (Table 1). In multivariate analysis, age, sex, reconstruction, BMI, tumor stage, location of tumor and DM were not independent risk factors for gastroparesis. Laparoscopic operation (HR, 2.731; P = 0.008) and less than 24 months between surgery and the gastric-emptying study (HR, 2.795; P = 0.001) were independent risk factors for gastroparesis after distal gastrectomy (Table 2). Laparoscopic gastrectomy, less than 24 months from surgery to gastric-emptying study and food stasis were significantly more frequent for patients whose gastric emptying was grade 2 compared to other grades (Table 3). Chronological analysis showed gastroparesis in 66.1% with 12 months between surgery and the gastric-emptying study, in 64.3% with less than 12–24 months, in 45.5% with 24–36 months, in 35.5% with 36–48 months, and in 43.8% with more than 48 months (P = 0.018). Gastroparesis after distal gastrectomy improved over time (Table 4, Fig. 1).
Distal gastrectomy is a common surgery for middle or lower gastric cancer. Distal gastrectomy is a severely destructive surgery to the anatomy of the stomach and its neighboring organs and has the possibility of leading to poor motility [9]. Gastric motility disorders may occur with distal gastrectomy, and this condition is responsible for poorer QoL before surgery than after distal gastrectomy [9]. Most patients with distal gastrectomy recover QoL, but some have poorer QoL than before surgery. QoL recovery after gastrectomy may be related to physiological changes in the remnant stomach. The enhancement of reservoir function and recovery of gastric motility can improve QoL after distal gastrectomy, but reservoir function and recovery of gastric motility are unknown. Our data showed a percentage of gastroparesis of 53.2%, and that it was a common condition in patients with distal gastrectomy. Although abnormal gastric emptying is not related to clinical dyspepsia [11], our data showed that delayed gastric-emptying grade in a gastric-emptying study was related to food stasis. In our study, patients with delayed gastric emptying more than 2 fold higher than the normal range showed significantly more food stasis. However, the condition was not severe enough to require medical or surgical intervention. We did not describe in the RESULTS section a patient with gastroparesis on gastric-emptying test who was readmitted because of delayed gastric emptying.
A time interval of less than 24 months between surgery and a gastric-emptying study was a risk factor for gastroparesis in univariate and multivariate analysis. The percentage of gastroparesis after distal gastrectomy tended to gradually decrease after 24 months and was significant. More than 2 years after surgery, greater than half of patients showed normal gastric empting times. The rate of gastroparesis increased slightly in patients more than 48 months after surgery, which was not described in the results, but because more patients underwent laparoscopic surgery. Few studies have reported chronological functional changes of the remnant stomach after gastrectomy. We propose that improved gastric emptying time may be related to adaptation of the remnant stomach and expansion of the anastomotic diameter. Our previous study showed that the relaxation phenomenon of the gastric fundus is highly enhanced in patients who undergo completion total gastrectomy for remnant stomach cancer compared to patients who undergo total gastrectomy. A direct complication of distal gastrectomy is loss of gastric reservoir function. However, gastric volume after distal gastrectomy partly recovers with proper alimentotherapy. As subtotal gastrectomy saves the gastric proximal region, this relaxation seems natural [12]. We propose that highly enhanced relaxation of the fundus of the remnant stomach may be related to chronological changes in gastroparesis after distal gastrectomy and, we assume, recovery of QoL after gastrectomy. A study of weight recovery after Roux-en-Y gastric bypass in patients with obesity may give insights into gastric function after distal gastrectomy. Abu Dayyeh et al. [13] reported that gastrojejunal anastomosis is significantly associated with weight regain after Roux-en-Y gastric bypass. They asserted that a dilated gastrojejunal anastomosis may induce early gastric emptying and loss of postprandial satiety. This anatomic alteration would present in the same manner as simple noncompliance. Our data showed that gastric-emptying time gradually improved after gastrectomy and was significantly related to food stasis. We propose that anastomotic dilatation after distal gastrectomy may be associated with gradual improvement in gastric emptying. Our study had the limitation that gastric-emptying studies were not performed at the same time interval in all patients, but were performed when patients visited our department during follow-up. We did not perform studies at the same time intervals in all patients because of cost and because most patients did not have clinical symptoms of delayed gastric emptying.
Laparoscopic distal gastrectomy was a risk factor for gastroparesis in univariate and multivariate analysis. Few studies have reported risk factors for gastroparesis after gastrectomy. Surgical techniques of open gastrectomy and laparoscopic distal gastrectomy have many differences such as tissue traction or using energy devices. We propose that laparoscopic energy-based devices may be responsible for gastroparesis after laparoscopic surgery. Laparoscopic energy-based devices can cause thermal injury to interstitial cells of Cajal that regulate gastric motility. This injury may cause gastric motility dysfunction after laparoscopic distal gastrectomy.
Gastroparesis was common on gastric scintigraphy after distal gastrectomy. Gastroparesis tended to improve after gastrectomy, which may be attributable to recovery of gastric motility and reservoir function. Our data showed that laparoscopic gastrectomy was an independent risk factor for gastroparesis. Therefore, laparoscopic gastrectomy should be performed with cautious handling of laparoscopic energybased devices to prevent gastroparesis.
Figures and Tables
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123.
2. Kim DH, Kim SM, Hyun JK, Choi MG, Noh JH, Sohn TS, et al. Changes in postoperative recurrence and prognostic risk factors for patients with gastric cancer who underwent curative gastric resection during different time periods. Ann Surg Oncol. 2013; 20:2317–2327.
3. Onate-Ocana LF, AielloCrocifoglio V, MondragonSanchez R, RuizMolina JM. Survival benefit of D2 lympadenectomy in patients with gastric adenocarcinoma. Ann Surg Oncol. 2000; 7:210–217.
4. Kim JP. Surgical results in gastric cancer. Semin Surg Oncol. 1999; 17:132–138.
5. Urushihara T, Sumimoto K, Shimokado K, Kuroda Y. Gastric motility after laparoscopically assisted distal gastrectomy, with or without preservation of the pylorus, for early gastric cancer, as assessed by digital dynamic x-ray imaging. Surg Endosc. 2004; 18:964–968.
6. Karanicolas PJ, Graham D, Gonen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013; 257:1039–1046.
7. Seok JW. How to interpret gastric emptying scintigraphy. J Neurogastroenterol Motil. 2011; 17:189–191.
8. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002; 5:83–89.
9. Lee HF, Chang FY, Lu CL, Luo JC, Chen CY, Wu HC. Electrogastrographic characteristics in subjects with stomach remnant. J Gastroenterol Hepatol. 2010; 25:339–344.
10. Kim KH, Kim MC, Jung GJ. Risk factors associated with delayed gastric emptying after subtotal gastrectomy with Billroth-I anastomosis using circular stapler for early gastric cancer patients. J Korean Surg Soc. 2012; 83:274–280.
11. Piessevaux H, Tack J, Walrand S, Pauwels S, Geubel A. Intragastric distribution of a standardized meal in health and functional dyspepsia: correlation with specific symptoms. Neurogastroenterol Motil. 2003; 15:447–455.
12. Kim DH, Kim YC, Choi W, Yun HY, Sung R, Kim HS, et al. High K(+)-Induced relaxation by nitric oxide in human gastric fundus. Korean J Physiol Pharmacol. 2012; 16:297–303.
13. Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011; 9:228–233.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download