Abstract
Purpose
With the increasing incidence of papillary thyroid microcarcinoma (PTMC), familial papillary thyroid microcarcinoma (FPTMC) is now recognized more frequently. However, the biological behavior of FPTMC is poorly understood. The aim of this study was to investigate the prevalence of FPTMC and its biological aggressiveness.
Methods
Between March 2006 and July 2010, 2,414 patients underwent primary surgical therapy for PTMC and 149 (6.2%) were further classified as FPTMC. To determine the biological aggressiveness of FPTMC, we compared the clinicopathological features and prognosis between FPTMC and sporadic PTMC (SPTMC).
Results
The male-to-female ratio was higher in FPTMC than in sporadic papillary thyroid microcarcinoma (SPTMC: 1:4.5 vs. 1:7.2, P = 0.041). The central lymph node (LN) metastasis rate was significantly higher in FPTMC than in SPTMC (36.2% vs. 24.2%, P = 0.002). The local recurrence rate was also higher in FPTMC than in SPTMC (4.5% vs. 0.6%, P < 0.001). We identified familial occurrence in 6.2% of cases of PTMC. FPTMC is associated with a high rate of central LN metastasis and local recurrence.
Papillary thyroid carcinoma (PTC) is the most common thyroid follicular cell-derived malignancy and generally comprises 85% of nonmedullary thyroid cancers (NMTC) [1]. Although most NMTC develops sporadically, it is known that 3.5%–6.2% of NMTC are familial in origin [2]. A familial nonmedullary thyroid cancer (FNMTC) is defined as a differentiated thyroid carcinoma that occurs in 2 or more first-degree relatives, including the index patient, without other familial syndromes [3]. Two manifestation patterns have been reported for FNMTC. The first pattern occurs as a minor component of familial cancer syndromes such as familial adenomatous polyposis, Gardner syndrome, and Cowden disease. The second pattern occurs in familial aggregates with no other familial syndrome [4].
Since Robinson and Orr [5] first reported a case of familial PTC in 1955 in 24-year-old identical twins, population studies have reported that the risk of thyroid cancer increases 9 fold in patients with a first-degree relative with thyroid cancer [6]. However, the disease aggressiveness of FNMTC is still under debate. Some studies have demonstrated that FNMTC has a less favorable prognosis compared to the sporadic form due to higher rates of multiplicity, lymph node (LN) metastases, and local invasion [378]. In contrast, other studies have showed that the biological behavior of FNMTC is not different from the sporadic form [910].
Papillary thyroid microcarcinoma (PTMC), defined as a PTC measuring 1.0 cm or less has been reported to be incidentally found in 6%–36% of autopsy studies [11]. PTMC patients have been classified as a low-risk group with excellent prognoses [12]. Diagnoses of PTMCs are increasing because of the increased use of high-resolution ultrasonography, which is capable of providing guided biopsies of nodules as small as 3 mm in diameter [13], as well as increased pathological detection in thyroid specimens removed for benign pathology [14]. With the increasing incidence of PTMC, familial papillary thyroid microcarcinoma (FPTMC) is becoming more common than previously expected. However, little is known about the biological behavior of FPTMC. Therefore, we investigated the prevalence of FPTMC, its biological aggressiveness, and outcome in comparison with sporadic papillary thyroid microcarcinoma (SPTMC).
We retrospectively reviewed the medical records of 2,414 consecutive patients who underwent initial surgical therapy for PTMC between March 2006 and July 2010 in the Department of Surgery, Yonsei University College of Medicine.
Data were obtained from a prospectively collected endocrine surgery database at our institution. This study was approved by the Institutional Review Board (approval number: 4-2013-0009).
We questioned patients on family history of thyroid disease when first evaluated in the outpatient clinic. PTMC patients who had one or more first-degree relative (parent, brother, sister, son, or daughter) with differentiated thyroid carcinoma were defined as having FPTMC. The criteria for FPTMC in this study were the same as those reported by Lupoli et al. [7]. PTMC patients with no family history were defined as sporadic disease.
Diagnosis of PTMC in all patients was preoperatively confirmed by an ultrasonographically guided fine needle aspiration biopsy (FNAB). If a small nodule suspicious of being malignant was present in the contralateral lobe, we performed additional FNAB for that nodule to determine the extent of thyroidectomy. If a lateral neck LN was suspected of metastasis, FNAB for the node and thyroglobulin (Tg) measurement in the wash-out of needles used for FNAB [15] were performed to determine whether the patient needed therapeutic modified radical neck dissection (MRND). Staging neck ultrasonography (US) and neck CT scans were used to evaluate preoperative clinical stages [16].
We excluded patients with a history of previous exposure to radiation or with evidence of familial cancer syndromes, such as familial adenomatous polyposis, Gardner syndrome, and Cowden disease. Patients with anaplastic carcinoma, medullary carcinoma, follicular carcinoma, malignant lymphoma, or metastatic carcinoma from other organs were not included in the present study.
The extent of thyroidectomy was determined based on preoperative findings, total thyroidectomy (TT) was indicated as follows: presence of contralateral thyroid nodules or multiplicity, presence of extrathyroidal invasion, or LN metastasis on preoperative ultrasonography. In these periods, the presence of family history was not regarded as an indication of TT. Therapeutic central compartment node dissection (CCND) was performed in patients with central LN metastasis on preoperative imaging study. Prophylactic ipsilateral CCND was performed in all patients without central LN metastasis on preoperative study. Therapeutic MRND was additionally performed in cases with metastases to lateral nodes.
After surgery, all patients were treated with levothyroxine (LT4, 2 µg/kg) to suppress thyroid-stimulating hormone. Patients who satisfied indications underwent radioactive iodine (RI) therapy without a diagnostic 131I whole-body scan in order to avoid a “stunning effect" (i.e., decreased uptake by a thyroid remnant of 131I after diagnostic administration of 131I) [17]. RI with 30–200 mCi was administered 4–6 weeks after TT, when each patient was in hypothyroidism after LT4 had been withdrawn for 4 weeks and a low-iodine diet had been maintained for 2 weeks. Patients received written instructions and were assisted by a dietician. The 1,31I whole body scan was taken on the second day after RI treatment. We regularly followed all patients by neck US and serum Tg at intervals of 6 or 12 months to examine whether there were any findings to indicate local recurrence. Either chest roentgenography or CT scan was also performed once per year to detect potential lung metastasis. The postoperative follow-up duration was median 67 months (range, 25–105 months). We considered a patient recurrent when recurrence was diagnosed by neck US or CT plus cytological examination (when necessary).
To determine the biological aggressiveness of FPTMC, we compared the clinicopathological features and prognosis between familial and sporadic PTMC. These clinicopathological parameters included demographic information, histological findings, extent of initial surgery, TNM stage [18], RI treatment, and incidence of recurrence.
All data are expressed as the mean ± standard deviation, proportions, or absolute numbers. We used IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) for statistical analyses. Statistical differences between the 2 groups were assessed by Student unpaired t-test and chi-square test. A P-value less than 0.05 was considered statistically significant. Recurrence-free survival (RFS) rate was based on the development of recurrent thyroid cancer. The Kaplan-Meier method with log-rank test was used to calculate RFS rates. Statistically significant variables based on univariate analysis were included in multivariate analysis using Cox regression test.
Of the 2,414 total patients, 149 (6.2%) were defined as having FPTMC and the remaining 2,265 (93.8%) were sporadic PTMC. All patients were identified as PTMC upon final pathology. Of these 149 FPTMC patients, 131 (87.9%) had 2 affected members in their family and the remaining 18 (12.1%) had 3 or more affected family members.
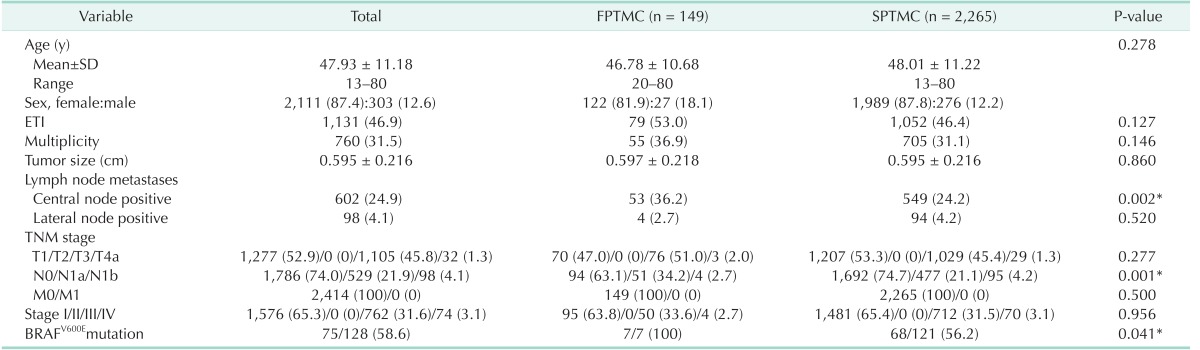
Clinicopathological findings of both groups are shown in Table 1. The 2 groups were well matched for age (P = 0.278). However, the female-to-male ratio was lower in FPTMC than in SPTMC (1:4.5 vs. 1:7.2, P = 0.041). There were no differences in pathological findings, except for central LN metastasis. The central LN metastasis rate was significantly higher in FPTMC than in SPTMC (36.2% vs. 24.2%, P = 0.002). Accordingly, there were no differences in TNM stage classification between the 2 groups, except for N1a stage. The rate of N1a was significantly higher in FPTMC than in SPTMC (34.2% vs. 21.1%, P = 0.001). In FPTMC, 95 patients (63.8%) had stage I disease, 50 (33.6%) had stage III, and 4 (2.7%) had stage IV. Similarly, in SPTMC, 1,481 patients (65.4%) had stage I disease, 712 (31.5%) had stage III, and 70 (3.1%) had stage IV. These results were not statistically significant.
The extent of thyroidectomy and LN dissection for patients with familial and sporadic PTMC is summarized in Table 2. TT was performed in 110 patients (73.8%) with FPTMC and in 1,506 patients (66.5%) with sporadic PTMC. MRND was performed in 4 patients (2.8%) with FPTMC and in 95 patients (4.1%) with SPTMC. However, there were no statistically significant differences in terms of thyroidectomy extent and LN dissection between the 2 groups.
The extent of thyroidectomy and LN dissection for patients with familial and sporadic PTMC is summarized in Table 2. TT was performed in 110 patients (73.8%) with FPTMC and in 1,506 patients (66.5%) with sporadic PTMC. MRND was performed in 4 patients (2.8%) with FPTMC and in 95 patients (4.1%) with SPTMC. However, there were no statistically significant differences in terms of thyroidectomy extent and LN dissection between the 2 groups.
After TT, 98 FPTMC patients (65.8%) and 1,302 SPTMC patients (57.5%) underwent low dose (30 mCi) RI therapy for remnant ablation (Table 1). Eight FPTMC patients (5.4%) and 119 SPTMC patients (5.3%) were treated with high dose (150–200 mCi) RI therapy due to T4 disease, extensive LN metastases. There was no significant difference in the rate of RI therapy between the 2 groups.
During follow-up, among the 2,414 patients, 2,103 patients (FPTMC: 133 patients/89.3%, SPTMC: 1,970 patients/87.0%) were followed up completely. The recurrence rate was higher in FPTMC than in SPTMC (4.5% vs. 0.6%, P < 0.001). All recurrences in both groups were not distant recurrences but local recurrences. In FPTMC, 2 patients experienced recurrence in the central neck node and 4 patients in the lateral neck node. In SPTMC, 2 patients experienced recurrence in the central neck nodes, 7 patients in the lateral neck node, 1 patient in the mediastina LN, and 1patient experienced recurrence on the remnant contralateral thyroid. As shown in Fig. 1, the RFS in patients with FPTMC was 98.5%, 97.6%, and 93.8% in 3, 5, and 7 years, respectively. And the RFS of SPTMC was 99.8%, 99.5%, and 99.5% in 3, 5, and 7 years, respectively (P < 0.001).
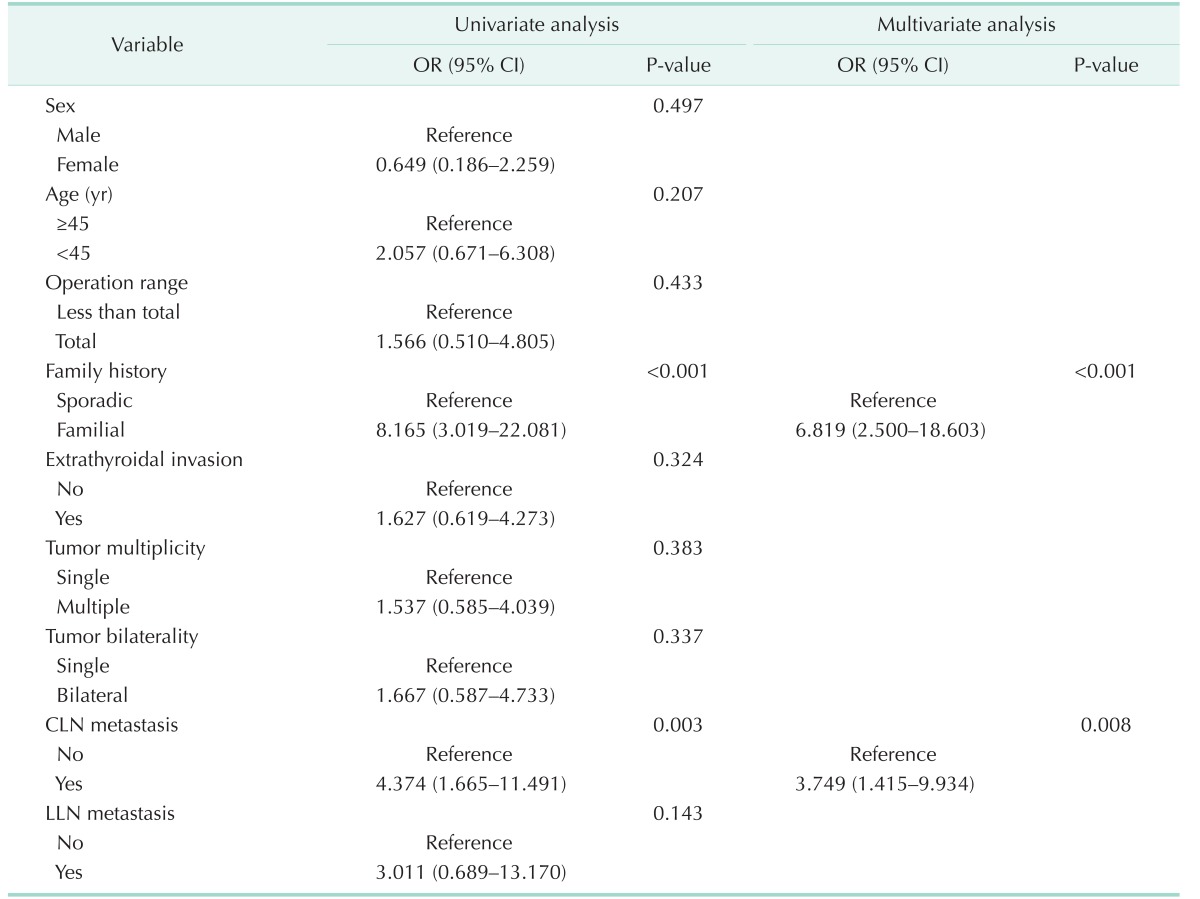
The risk factors for recurrence were evaluated using univariate and multivariate logistic regression analyses for all study populations (Table 3). The multivariate analyses revealed that presence of familial history and central LN metastasis were independent risk factors for recurrence. Interestingly, the extent of thyroidectomy was not significantly associated with recurrence in our study population.
To our knowledge, this is the first large study evaluating the prevalence, biological behavior, and outcome of FPTMC versus SPTMC. We found that the prevalence of FPTMC among PTMC cases was 6.2%, which was similar to that of FNMTC, which ranges from 3.5% to 6.2% [219]. Regarding FPTMC biological behavior, the present study found that FPTMC had a higher incidence in male patients, central LN metastasis, and recurrence compared to SPTMC. Consequently, the DFS of FPTMC patients was lower than that of SPTMC patients, despite the short follow-up periods in this study.
Male sex, one of the conventional prognostic factors, has been reported to be a risk factor for central LN metastasis in PTMC patients [2021]. Thus, our result of increased male sex in FPTMC is considered a finding that represents the aggressive behavior of FPTMC.
As the results of the present study correspond with the results of central LN involvement in PTMC, which are significantly associated with recurrence [1222], we routinely performed CCND in PTMC patients. We found that FPTMC patients had more aggressive disease behavior than SPTMC patients due to a higher rate of central LN metastases, as FNMTC patients are more likely to have LN metastasis [22]. A large study of 1,066 PTMC patients by Zhang et al. [21] recommended that prophylactic CCND should be considered in PTMCC patients presenting risk factors. On the basis of these data and our findings, we cautiously suggest that prophylactic CCND is recommended in the management of FPTMC patients.
Unlike FNMTC, which has higher rates of multiplicity and extrathyroidal extension [922], there was no significant difference in the rate of multiplicity and extrathyroidal extension between the 2 groups in this study. However, considering that the absolute percentage of multiplicity and extrathyroidal extension in this study were higher in FPTMC than in SPTMC, a larger study of FPTMC patients remains necessary to elucidate whether multiplicity and extrathyroidal extension in FPTMC are aggressive characteristics. Previous studies demonstrated that the number of affected family members predicts a worse prognosis for the patient [823]. In this study, the prognosis of patients with 2 affected family members did not differ from patients with 3 or more affected family members (data not shown). A possible explanation for this finding is that there were only 18 patients with 3 or more affected members in this study. Therefore, further study is also necessary to confirm this finding.
Lupoli et al. [7] reported that FPTMC showed a higher recurrence rate than sporadic PTMC (57% vs. 4.5%) and requires more aggressive treatment. However, this finding was not confirmed by others because of the small numbers of FPTMC patients in their studies. Our relatively large study confirmed a higher recurrence rate and a lower 5-year RFS in FPTMC (97.6% vs. 99.5% in SPTMC), which is consistent with the study by Lupoli et al. [2425].
Although the treatment strategy for FNMTC is still debated, most studies suggest an aggressive treatment and careful follow-up for FNMTC patients rather than conservative management [1378]. Creach et al. [26] demonstrated that PTMC patients with LN metastases who did not receive RI therapy had a 5-year RFS of 42.9% versus 93.2% for patients who received RI therapy. They therefore recommended RI therapy for PTMC patients, particularly in patients with LN metastases. Another controversy exists regarding the treatment strategy of PTMC. The Mayo Clinic reviewed 900 patients with PTMC presenting a period of over 60 years with a mean follow-up time of 13.5 years [12]. The data did not support an aggressive approach for all PTMC patients as advocated by others because TT and postoperative RI therapy did not demonstrate a superior outcome for PTMC patients [27]. Lee et al. [28] noted that the long-term follow-up data of PTMC patients according to the operation method (less than total vs. TT) showed similar results. And in our results, the extent of thyroidectomy did not show significant correlation with the prognosis of PTMC patients. Thus, management of PTMC continues to be an area of controversy, and there is still no strong evidence for a standard treatment of FPTMC.
Although our indication for thyroidectomy in this study follows the recent American guidelines, the number of recurrence cases was too small to determine the influence of initial surgery and postoperative RI therapy on recurrence rates in either group [29], Furthermore, it is difficult to determine whether the higher rate of recurrence in this study is due to the natural history of FPTMC or an inappropriate FPTMC treatment protocol. Therefore, large-scale trials and long-term follow-up data are required to confirm the impact of aggressive approaches on recurrence in FPTMC patients. At present, a reasonable surgical strategy for FPTMC is that all primary tumors are completely resected at the same time (including tumor-bearing nodules in the contralateral lobe), and the central compartment nodes, the most frequent metastatic site, are adequately dissected and removed without perioperative morbidity.
Many previous articles reported that BRAF, NRAS, KRAS, RET/PTC, NTRK genes are associated with familial PTC [430]. Moses et al. [2] reported that there is no difference in the positive rate and distribution of somatic mutations between sporadic and familial type in clinical finding. In our study, the BRAFV600E mutation result is significantly different between FPTMC and SPTMC. However, we could check the BRAF mutation only in a small sample size, so the aggressiveness of FPTMC associated BRAF mutation remain unclear. Therefore, large-scale trials and long-term follow-up data are required to confirm our findings before definitive conclusions can be made.
In conclusion, we identified familial occurrence in 6.2% of cases of PTMC. FPTMC is associated with a high rate of central LN metastases and local recurrence. These findings suggest that close follow-up would be necessary especially in FPTMC patients to detect local recurrence.
ACKNOWLEDGEMENTS
This study was supported by a research grant of Yonsei University Academic-industrial cooperation (Dong-A ST) for 2015-31-0402.
References
1. Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007; 31:924–933. PMID: 17429563.

2. Moses W, Weng J, Kebebew E. Prevalence, clinicopathologic features, and somatic genetic mutation profile in familial versus sporadic nonmedullary thyroid cancer. Thyroid. 2011; 21:367–371. PMID: 21190444.

3. Grossman RF, Tu SH, Duh QY, Siperstein AE, Novosolov F, Clark OH. Familial nonmedullary thyroid cancer. An emerging entity that warrants aggressive treatment. Arch Surg. 1995; 130:892–897. PMID: 7632152.

4. Kebebew E. Hereditary non-medullary thyroid cancer. World J Surg. 2008; 32:678–682. PMID: 18058169.

5. Robinson DW, Orr TG. Carcinoma of the thyroid and other diseases of the thyroid in identical twins. AMA Arch Surg. 1955; 70:923–928. PMID: 14375516.

6. Charkes ND. On the prevalence of familial nonmedullary thyroid cancer in multiply affected kindreds. Thyroid. 2006; 16:181–186. PMID: 16613533.

7. Lupoli G, Vitale G, Caraglia M, Fittipaldi MR, Abbruzzese A, Tagliaferri P, et al. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet. 1999; 353:637–639. PMID: 10030330.

8. Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002; 26:897–902. PMID: 11965446.

9. Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009; 145:100–105. PMID: 19081481.

10. Maxwell EL, Hall FT, Freeman JL. Familial non-medullary thyroid cancer: a matched-case control study. Laryngoscope. 2004; 114:2182–2186. PMID: 15564841.

11. Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998; 83:553–559. PMID: 9690549.
12. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008; 144:980–987. PMID: 19041007.

13. Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007; 3:240–248. PMID: 17315032.

14. Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008; 144:1038–1043. PMID: 19041015.

15. Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005; 29:483–485. PMID: 15776292.

16. Park JS, Son KR, Na DG, Kim E, Kim S. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009; 192:66–72. PMID: 19098181.

17. Morris LF, Waxman AD, Braunstein GD. Thyroid stunning. Thyroid. 2003; 13:333–340. PMID: 12804101.

18. Sobin L, Wittekind CH. TNM: classification of malignant tumours. 6th ed. New York: Wiley-Liss;2002.
19. Pal T, Vogl FD, Chappuis PO, Tsang R, Brierley J, Renard H, et al. Increased risk for nonmedullary thyroid cancer in the first degree relatives of prevalent cases of nonmedullary thyroid cancer: a hospital-based study. J Clin Endocrinol Metab. 2001; 86:5307–5312. PMID: 11701697.

20. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010; 148:526–531. PMID: 20189620.

21. Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. 2012; 97:1250–1257. PMID: 22319042.

22. Mazeh H, Benavidez J, Poehls JL, Youngwirth L, Chen H, Sippel RS. In patients with thyroid cancer of follicular cell origin, a family history of nonmedullary thyroid cancer in one first-degree relative is associated with more aggressive disease. Thyroid. 2012; 22:3–8. PMID: 22136209.

23. Triponez F, Wong M, Sturgeon C, Caron N, Ginzinger DG, Segal MR, et al. Does familial non-medullary thyroid cancer adversely affect survival? World J Surg. 2006; 30:787–793. PMID: 16479341.

24. Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004; 89:3713–3720. PMID: 15292295.

25. Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006; 91:2171–2178. PMID: 16478817.

26. Creach KM, Siegel BA, Nussenbaum B, Grigsby PW. Radioactive iodine therapy decreases recurrence in thyroid papillary microcarcinoma. ISRN Endocrinol. 2012; 2012:816386. PMID: 22462017.

27. Cappelli C, Castellano M, Braga M, Gandossi E, Pirola I, De Martino E, et al. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol. 2007; 95:555–560. PMID: 17226813.

28. Lee J, Park JH, Lee CR, Chung WY, Park CS. Long-term outcomes of total thyroidectomy versus thyroid lobectomy for papillary thyroid microcarcinoma: comparative analysis after propensity score matching. Thyroid. 2013; 23:1408–1415. PMID: 23509895.

29. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142. PMID: 16420177.

30. McKay JD, Lesueur F, Jonard L, Pastore A, Williamson J, Hoffman L, et al. Localization of a susceptibility gene for familial nonmedullary thyroid carcinoma to chromosome 2q21. Am J Hum Genet. 2001; 69:440–446. PMID: 11438887.

Fig. 1
Kaplan-Meier curve for recurrence free survival of familial (FPTMC) and sporadic papillary thyroid microcarcinoma (SPTMC).
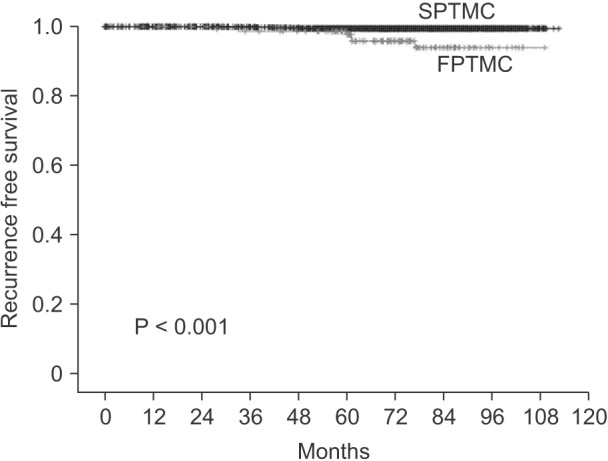
Table 2
Extent of surgery and postoperative radioactive iodine therapy in both groups
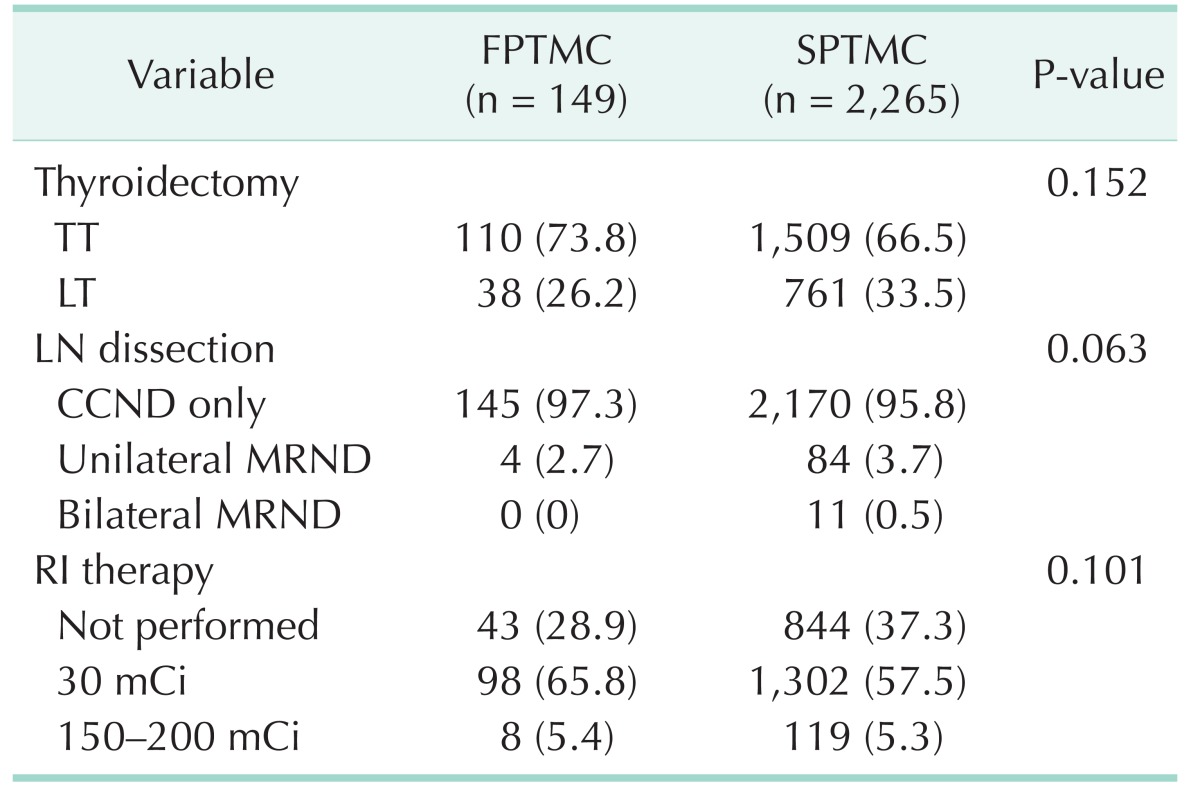
Values are presented as number (%).
FPTMC, familial papillary thyroid microcarcinoma; SPTMC, sporadic papillary thyroid carcinoma; TT, total thyroidectomy; LT, less than total thyroidectomy; LN, lymph node; CCND, central compartment node dissection; MRND, modified radical neck dissection; RI, radioactive iodine.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download