Abstract
Purpose
Previous studies have shown the role of Sal-like protein 4 (SALL4) as a biomarker in hepatocellular carcinoma (HCC), and some studies have shown the relationship between SALL4 and prognosis. Given the debates in study groups differences in terms of etiologic causes between Western and Asian HCC and detection methods, we attempted to verify the features of SALL4 immunoreactivity and its clinical correlation in Korean HCC patients.
Methods
Immunohistochemical staining of SALL4 of tissue microarrays (TMAs) consisting of 213 surgically resected HCC patients' tissue were scored in a semiquantitative scoring system with immunoreactive score and the results analyzed with clinical outcome, in addition to general demographics and clinical characteristics.
Results
SALL4 immunoreactivity was expressed in 50 cases. Relevance between SALL4 and α-FP correlated significantly (P = 0.002). Also, the SALL4-positive patients had considerably higher tumor grade (P < 0.001). The survival analysis showed negative correlation with SALL4 immunoreactivity in all HCC patient groups, but SALL4 immunoreactivity in T3 and T4 HCC correlated with poor prognosis.
Hepatocellular carcinoma (HCC) is a universal malignant tumor with poor prognosis. It is the third cause of cancer mortality after lung cancer and stomach cancer globally, also in Korea [1]. Although there have been significant developments in therapies for HCC including surgery, transarterial chemoembolization, radiofrequency thermal ablation, and multikinase inhibitors (sorafenib, sunitinib, etc.), HCC remains a lethal disease [23]. Accordingly, discovery of new therapeutic targets and biomarkers for early diagnosis and prognosis prediction of HCC with development of gene and expression analysis comes to the forefront [456].
Sal-like protein 4 (SALL4) is a human homolog to Drosophaila spalt. In humans, SALL4 is one of several transcription factors which are a valuable part of the self-renewal of embryonic stem cells as well as in embryonic development [789]. Many published studies showed SALL4 is an oncogene in leukemia and other various types of cancers like non–small-cell lung cancers (NSCLC), breast cancer, and testicular germ cell tumors [101112]. In the liver, elevated SALL4 was found in fetal liver, and SALL4 gradually diminishes during development [13]. Yong et al. [14] showed unique expression patterns of SALL4 in human livers at various stages – expressed as an oncofetal protein in HCC, activated in liver of fetus, and inactivated in liver of adult.
Recently, outstanding studies have suggested SALL4, an oncofetal gene, as a prognosis biomarker in HCC. With that, previous studies that were published showed SALL4 expression in HCC patients could potentially be a new treatment target for HCC. However, there were many debates in study groups on the difference in terms of etiologic causes between Western and Asian HCC and detection method. Hence, general application of SALL4 for HCC is needed to confirm the diagnostic and therapeutic efficacy of SALL4 [14151617].
Therefore, we tried to verify the features of SALL4 immunoreactivity and its clinical correlation in Korean HCC patients. We will use SALL4 as a new therapeutic target for HCC based on this study.
A total of 213 patients with HCCs with complete clinicopathologic data were collected. Patients were underwent curative surgery at the department of surgery, Hanyang University Seoul Hospital between January 1991 and September 2013. Medical records were reviewed and characteristic data (age, sex), pathologic data (tumor size, tumor number, histologic grade, vascular invasion, perineural invasion), and clinical data (α-FP, survival, ascite, albumin, bilirubin, encephalopathy, prothrombin time) were extracted.
The pathologist (S.S.P.) reviewed the histology of each case, and selected the representative, properly fixed, and viable areas for tissue microarray (TMA) construction. Single 2.0-mm tissue cores were sampled from donor blocks and transferred to recipient blocks.
The 4-µm-thick tissue sections from TMA blocks were deparaffinized with xylene and rehydrated in a graduated series of ethanol. For heat-induced antigen retrieval, the following conditions were applied for optimization: autoclave heating at 100℃ for 30 minutes; and microwave heating for 12 minutes in pH 6.0 sodium citrate buffer. 3% H2O2 was used for blocking endogenous peroxidase activity. TMA slides were incubated with anti-SALL4 antibody (dilution factor 1:200, clone 6E3, Sigma-Aldrich, St Louis, MO, USA) at 4℃ overnight, and then incubated with antirabbit secondary antibody (Dako, Carpinteria, CA, USA) at room temperature for 30 minutes. 3,3´-Diaminobenzidine was used as a chromogen for visualization.
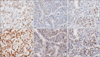
The immunohistochemical staining slides were interpreted by 2 pathologists who were blinded to clinical data. The interpretation was based on a semiquantitative system; the staining intensity and proportion. Staining intensity was scored as 0 (negative), 1 (weak), 2 (intermediate), 3 (strong). Staining proportion was scored as 0 (<10%), 1 (11%–25%), 2 (26%–50%), 3 (51%–75%), 4 (76%–100%) according to the percentage of immunoreactive tumor cells. The final score as immunoreactive score (IRS) was calculated by multiplying intensity score by proportion score (Fig. 1). xPositive SALL4 expression was defined as tumor cells having nuclear staining with minimal background and 1 or more IRS.
The appropriate statistical analysis was used with IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). The correlation between the SALL4 expression and tumor size, α-FP was evaluated using Student t-test. The chi-square test was used to analyze the relationship between SALL4 expression and the clinical and pathological characteristics, including patient sex, histologic grade, American Joint Committee on Cancer (AJCC) stage, Child-Pugh score, Okuda stage, perineural invasion, and blood vessel invasion. The survival analyses were obtained by Kaplan-Meier methods, and log-rank test was used to compare their differences. A difference of P-value less than 0.05 between groups was considered significant.
Table 1 summarizes the general characteristics of HCC patients. Of the 213 patients who were included in this TMA analysis, 167 patients (78.4%) were male and 46 patients (21.6%) were female. The mean age was 55.39 years (range, 15–87 years) and the mean survival was 49.97 months (range, 0–278 months). The average tumor size of the largest tumor in patients were 4.76 cm (range, 0.7–22 cm) and the majority (n = 176, 82.6%) of patients had single tumor. The mean serum α-FP level was 715.11 ng/mL (range, 1–11,000 ng/mL).
Table 2 summarizes the clinicopathologic characteristics of HCC patients. Among 213 patients with available tumor grading information (Edmondson and Steiner Grade), 13 patients (6.1%) were grade 1, 74 patients (34.7%) were grade 2, 101 patients (47.4%) were grade 3, and 19 patients (8.9%) were grade 4. The Child-Pugh score was marked for all patients who were included in this study, among whom, 200 patients (93.9%) were stage A, and the other 13 patients (6.1%) were stage B. The AJCC staging information was in use for 211 patients: 119 patients (55.9%) were stage I, 45 patients (21.1%) were stage II, 40 patients (18.8%) were stage III, and 7 patients (3.3%) were stage IV. The Okuda stage was available for all patients: 175 patients (82.2%) were stage I, and the other 38 patients (17.8%) were stage II. Vessel invasion was seen in 86 patients (40.4%), and perineural invasion was seen in 5 patients (2.3%).
Using the semiquantative scoring system for SALL4 as described above, among 213 HCC TMA specimens, 50 (23.5%) showed scores of ≥1 (range, 1 to 9). According to the method of antigen retrieval, there was difference in staining intensity; Autoclave heating at 100oC for 30 minutes (50 cases) increased the positive expression cases than heating for 12 minutes (2 cases) in sodium citrate buffer (Fig. 2).
It demonstrated that the SALL4-positive HCCs had considerably higher tumor grade (P < 0.001) compared with SALL4-negative HCCs. On the clinicopathologic analyses, relevance between SALL4 and clinicopathologic features were not found in microscopic and macroscopic vessel invasions, perineural invasions, but SALL4 and α-FP correlated significantly (P = 0.002) (Table 2).
Following the univariate analysis, SALL4 immunoreactivity was associated with prognosis. There was no considerable correlation with SALL4 and prognosis. However, survival analysis showed positive correlation with largest tumor size (P = 0.005), and SALL4 immunoreactivity in T3 and T4 HCC were correlated with poor prognosis (Fig. 3).
HCC is a lethal disease with a high mortality among various cancers [18]. In this study, we show the features of SALL4 immunoreactivity and its clinical correlation in Korean HCC patients.
SALL4 immunoreactivity (defined as IRS ≥1) was seen in 50 cases of a total 213 cases in our analysis. In this study, histologic grade and α-FP were correlated significantly with SALL4. Correlations between SALL4 and clinicopathologic factors were not seen in AJCC stage, Okuda stage, vessel invasion, perineural invasion, or tumor size. In the survival analysis, positive correlation with large tumor size, histologic grade, and SALL4 immunoreactivity in T3 and T4 were correlated with poor prognosis.
From these results, we have several significant clinical suggestions. SALL4 immunohistochemisty correlates with aggressive progress and poor prognosis of HCC in some cases. Therefore, it shows the potency of SALL4 as a prognostic biomarker in HCC, and furthermore as a therapeutic target for HCC.
However, there are some limitations in this study. First, the TMA of HCC in this study consisted of resected specimens of HCC patients from only one center in Korea without a database of underlying disease. Some studies published previously showed that there were differences of SALL4 expression according to ethnicity or underlying disease like HBV [1415]. Second, immunohistochemical staining method is slightly different from previous studies in areas of antigen retrieval and so on. Also, interpretation of SALL4 expression includes injection of pathologists' subjectivity. Any ideal prognostic or therapeutic biomarker should be performed easily with accurate and simple methods and have high sensitivity and specificity. From now on, the objectification of SALL4 expression is needed with further study.
In summary, we found that positive immunostaining of SALL4 is correlated with poor patient survival outcome in large and undifferentiated Korean HCC. SALL4 expression showed close relationship with clinical outcomes of HCCs in Korean patients. Further careful well-designed study for SALL4 stem cell marker as a biomarker for HCCs should be done with multinational studies for general application of SALL4.
Figures and Tables
Fig. 1
The representative sections of Sal-like protein 4 immunohistochemical staining in hepatocellular carcinoma. The nuclei of tumor cells shows negative (A), intermediate (B), and strong (C) immunoreactivity. No hepatocellular carcinoma revealed strongly positive immurnostaining, which is shown in germ cell tumor (×400).

Fig. 2
Comparison of Sal-like protein 4 immunostaining for different antigen retrieval methods. Strong positive staining in the nuclei of tumor cells of germ cell tumor (positive control) is observed in section treated with 30-minute autoclave heating (D), whereas intermediate staining is observed in the consecutive section treated with 12-minute microwave heating (A). Two representative sections from hepatocellular carcinoma also shows an enhanced immunoreactivity in sections treated with 30-minute autoclave heating (E, F), compared to 12-minute microwave method (B, C) (×400).
ACKNOWLEDGEMENTS
These work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01100202)" Rural Development Administration, Republic of Korea.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
2. Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008; 48:1312–1327.
3. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
4. Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007; 45:938–947.
5. Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006; 131:1758–1767.
6. Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011; 140:1501–1512.e2.
7. Yang J, Gao C, Chai L, Ma Y. A novel SALL4/OCT4 transcriptional feedback network for pluripotency of embryonic stem cells. PLoS One. 2010; 5:e10766.
8. Rao S1, Zhen S, Roumiantsev S, McDonald LT, Yuan GC, Orkin SH. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol Cell Biol. 2010; 30:5364–5380.
9. Ma Y, Cui W, Yang J, Qu J, Di C, Amin HM, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006; 108:2726–2735.
10. Kobayashi D, Kuribayshi K, Tanaka M, Watanabe N. SALL4 is essential for cancer cell proliferation and is overexpressed at early clinical stages in breast cancer. Int J Oncol. 2011; 38:933–939.
11. Fujimoto M, Sumiyoshi S, Yoshizawa A, Sonobe M, Kobayashi M, Moriyoshi K, et al. SALL4 immunohistochemistry in non-small-cell lung carcinomas. Histopathology. 2014; 64:309–311.
12. Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009; 33:1065–1077.
13. Oikawa T, Kamiya A, Kakinuma S, Zeniya M, Nishinakamura R, Tajiri H, et al. Sall4 regulates cell fate decision in fetal hepatic stem/progenitor cells. Gastroenterology. 2009; 136:1000–1011.
14. Yong KJ, Gao C, Lim JS, Yan B, Yang H, Dimitrov T, et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013; 368:2266–2276.
15. Liu TC, Vachharajani N, Chapman WC, Brunt EM. SALL4 immunoreactivity predicts prognosis in Western hepatocellular carcinoma patients but is a rare event: a study of 236 cases. Am J Surg Pathol. 2014; 38:966–972.
16. Han SX, Wang JL, Guo XJ, He CC, Ying X, Ma JL, et al. Serum SALL4 is a novel prognosis biomarker with tumor recurrence and poor survival of patients in hepatocellular carcinoma. J Immunol Res. 2014; 2014:262385.
17. Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, Yamazaki Y, et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology. 2013; 57:1469–1483.
18. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download