Abstract
A case of adrenal ganglioneuroblastoma is presented here. This adrenal ganglioneuroblastoma was found in a 27-year-old female 7 months after delivery. CT clarified that the tumor originated retroperitoneally and was large in size (11.4 cm × 9.4 cm). The tumor was surgically removed together with pancreatic body and tail, left kidney and spleen, and pathological diagnosis was adrenal ganglioneuroblastoma-intermixed. Adrenal ganglioneuroblastoma is extremely rare in adults, with only about 9 cases documented including this case.
Ganglioneuroblastoma generally occurs more often in children than in adults. It usually affects the posterior mediastinum, retroperitoneum and adrenal gland. Symptoms of ganglioneuroblastoma are not typical; most are a result of the local expanding-mass effect. Some even present based on the manifestation of metastasis. In this female adult, it presented an atypical swelling pain on the left side of the waist and abdomen, with intermittent and concurrent fever and headache. To some extent, pregnancy delayed the diagnosis of this case. Regular prenatal examination didn't show any abnormalities. The tumor was not found until symptoms presented 7 months after delivery. A huge mass was revealed by CT in retroperitoneum and its unclear boundary persuaded us to resect it surgically. The tumor was pathologically defined as ganglioneuroblastoma originating from the adrenal gland. A case of adrenal ganglioneuroblastoma-intermixed in female adults is reported as below. This research also reviews adrenal ganglioneuroblastoma in adults based on published literature.
A 27-year-old woman who was 7 months into her pregnancy was referred to the Department of Urinary Surgery at The First Hospital of Jilin University on 3th, April. She had suddenly felt continuously swelling pain on the left side of the waist and abdomen accompanied by fever one day earlier. The pain was alleviated without intervention. She went to her local hospital when the symptoms presented again more aggravated than before. Since CT showed a retroperitoneum mass, she was referred to our hospital.
On admission, her blood pressure was 108/62 mmHg. Physical examination showed left upper-quadrant tenderness, left renal area tenderness and knocking pain. There were no palpable abdominal masses or superficial lymph nodes. No endocrinological disorders were presented. Laboratory findings were as follows: urinalysis showed urine ketone bodies (KET) was 3+; peripheral blood examination showed white blood cell count of 11.24×109/L with a neutrophilic granulocyte percentage of 0.76 and a lymphocytes percentage of 0.11. Blood biochemistry showed a glucose value of 6.93 mmol/L. Coagulation examination showed a PT of 14.4 seconds and a international normalized ration of 1.23. Tumor marker showed neuronspecific enolase at 289.46 ng/mL. The systemic blood concentration of adrenalin, noradrenaline, and dopamine was 10.5 pg/mL, 38.6 pg/mL, and 13.6 pg/mL, respectively. The concentration of adrenocorticotrophic hormone was 5.76. While the concentration of aldosterone (clinostatism) was 13.6 pg/mL, aldosterone (8:00 AM) was 745.03 mmol/L. Twenty-four-hour urinary sample for vanilmandelic acid (VMA) was not possible to collect. All the endocrinological results mentioned above were in the normal range, except for the aldosterone (8:00 AM). Electrocardiography showed sinus tachycardia and brain CT was normal. Renal CT (Fig. 1) revealed a large (11.4 cm × 9.4 cm), oval and cyst-solid mixed tumor with slightly indistinct margins and septation in it. It was located in the left retroperitoneum close to the left kidney superiorly, the pancreatic body and tail posteriorly, and the spleen interiorly. In contrast-enhanced scan, solid portion of the tumor suggested enhancement while the cyst portion had no enhancement. There was no clear boundary between the spleen and the tumor, the left kidney and the left kidney vein were pressed and the fat gap around the tumor was turbid.
The possible diagnosis of malignancy and the pressure appearance of the surrounding organs compelled us to remove the tumor surgically. Given the fact that the present patient had no expression of hypertension or other cardiovascular disorders, (while only slight endocrinological abnormalities were present), there was a small chance that the tumor was pheochromocytoma. Therefore, in case it was atypical pheochromocytoma, the necessary preoperative preparation was undertaken. Starting from the three days before surgery, 500-mL voluven and 500-mL saline were given by intravenous injection once a day while 10-mg phenoxybenzamine hydrochloride was administered by mouth three times a day.
Due to the close connection between the tumor and surrounding organs, the tumor cannot be easily resected en bloc. Pancreatic body and tail, left kidney and spleen were removed along with the tumor in order to assure complete resection of the tumor.
Gross examination (Fig. 1) of the resected specimens revealed an 11 × 9 × 7.8-cm nodular tumor, the cut surface of the spleen, the pancreas and the left kidney had no apparent changes. There was some adrenal gland tissue on the margin of the tumor. The tumor was solid, soft nature, grey yellow or tawny in color and had a complete capsule. Histopathologically, the tumor turned out to be adrenal ganglioneuroblastoma-intermixed. There was extensive hemorrhage and necrosis with focal calcification inside the tumor tissue. Vessels and nerves were not infiltrated with the tumor cells, likewise the excised pancreas, spleen and kidney tissues. Histologic examination showed neuroblasts and gangliocytes (Fig. 2A). Immunohistochemistry showed that CgA, Syn, and NF were positive (Fig. 2B) while CD99 and Desinin were negative. Because of no effectiveness of follow-up treatment was reported, no adjuvant chemotherapy or radiation was undergone for our patient. Five months after operation, the patient remained healthy and there were no evidence of recurrence and metastasis.
Ganglioneuroblastoma is a neuroblastic tumor that arises wherever sympathetic tissue exists, mostly in adrenal medulla, extra-adrenal retroperitoneum, and posterior mediastinum [1]. Ganglioneroblastoma is composed of both mature gangliocytes and immature neuroblasts and has intermediate malignant potential [2]. These tumors are more common in childhood rather than in adults. To our knowledge, only 9 cases of adrenal ganglioneuroblastoma in adults including the present case have been reported.
No typical symptoms are presented in ganglioneuroblastoma. In some cases, the pre-existing symptoms are a result of the local expanding-mass effect, as in the present case [3]. Some cases may be found incidentally [45]. While some even develop into metastasis to other organs [67].
Ganglioneuroblastoma is generally subclassified into intermixed and nodular types [8]. The prognosis of ganglioneuroblastoma is somewhat determined by the malignant component in it. However, there are no clear delineations between neuroblastoma and ganglioneuroblastoma, and between ganglioneuroblastoma and ganglioneuroma [8]. According to The International Neuroblastoma Pathology Classification, neuroblastic tumors are divided into favorable and unfavorable groups. Ganglioneuroblastoma-intermixed tumor is classified into favorable group, as in our case, and ganglioneuroblastoma-nodular tumor is classified into favorable or unfavorable group based on the characteristics of the neuroblastic component. On the other hand, it is based on the age-linked evaluation of grade of neuroblastic differentiation and mitosis-learyorrhexis index of the neroblastomatous nodule(s). It is believed that complete resection and negative margins would mean a good prognosis for ganglioneuroblastoma. Some reports suggested ganglioneuroblastoma in adults are slow growing [9]. And adjuvant therapy may not be necessary after surgery [7]. Surgical excision of localized disease appears to be the only curative therapeutic regimen in adult ganglioneuroblastoma [10].
In the present patient, complete tumor resection was performed, and there was no evidence of metastasis. So our patient's prognosis is considered as favorable. However, further follow-up still be needed to support this conclusion.
Figures and Tables
Fig. 1
Computed tomographic images and gross examination of tumor. (A) Arterial phase, (B) venous phase, (C) delayed phase, and (D) gross pathologic specimens.
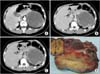
Fig. 2
Histologic examination and Immunohistochemistry of resected specimen. (A) Gangliocytes and neuroblasts showed in tumor (H&E): (1) gangliocytes (×200), (2) gangliocytes (×400), (3) neuroblasts (×200), (4) neuroblasts (×400); (B) CgA, Syn, and NF expressed in tumor (Immunohistochemistry): (1) CgA (magnification, ×400), (2) Syn (magnification, ×400), and (3) NF (magnification, ×400).

References
1. Morris JA, Shcochat SJ, Smith EI, Look AT, Brodeur GM, Cantor AB, et al. Biological variables in thoracic neuroblastoma: a Pediatric Oncology Group study. J Pediatr Surg. 1995; 30:296–302.
2. Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologicpathologic correlation. Radiographics. 2002; 22:911–934.
3. Yamanaka M, Saitoh F, Saitoh H, Nisimura S, Sawada Y, Tsukui A, et al. Primary retroperitoneal ganglioneuroblastoma in an adult. Int J Urol. 2001; 8:130–132.
4. Koike K, Iihara M, Kanbe M, Omi Y, Aiba M, Obara T. Adult-type ganglioneuroblastoma in the adrenal gland treated by a laparoscopic resection: report of a case. Surg Today. 2003; 33:785–790.
5. Hiroshige K, Sonoda S, Fujita M, Takasugi M, Kuroiwa A, Inatomi H. Primary adrenal ganglioneuroblastoma in an adult. Intern Med. 1995; 34:1168–1173.
6. Bacher U, Christopeit M, Wiedemann B, Leuschner I, Haferlach T, Choschzick M. Ganglioneuroblastoma infiltrating the bone marrow in an adult. Br J Haematol. 2011; 153:544.
7. Fujiwara T, Kawamura M, Sasou S, Hiramori K. Results of surgery for a compound adrenal tumor consisting of pheochromocytoma and ganglioneuroblastoma in an adult: 5-year follow-up. Intern Med. 2000; 39:58–62.
8. Okamatsu C, London WB, Naranjo A, Hogarty MD, Gastier-Foster JM, Look AT, et al. Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: a report from the CCG and COG. Pediatr Blood Cancer. 2009; 53:563–569.
9. Kilton LJ, Aschenbrener C, Burns CP. Ganglioneuroblastoma in adults. Cancer. 1976; 37:974–983.
10. Vaughan DE Jr, Blumenfeld JD. The adrenals. In : Walsh PC, Retik AB, Stamey TA, Vaughan DE, editors. Campbell's urology. 6th ed. Philadelphia: W.B. Saunders;1992. p. 2376.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download