Abstract
Purpose
The aim of this study was to evaluate the severity of disease in children with acute bronchiolitis according to the type of infected virus.
Methods
From November 2007 to May 2015, 768 patients under 2 years of age who underwent real time-polymerase chain reaction of nasopharyngeal aspirates admitted to the Department of Pediatrics of Dongguk University Ilsan Hospital for acute bronchiolitis were enrolled. Severe bronchiolitis was defined as presence of one or more kinds among tachypnea, chest retraction, needs of O2 inhalation or ventilator care.
Results
The severity of bronchiolitis was increased with shorter fever duration (P <0.001) and previous wheezing episodes (P =0.005). In the case of single infection, respiratory syncytial virus (RSV) A only increased the severity of acute bronchiolitis (P =0.012). However, the severity of illness decreased when RSV A coinfected with adenovirus (P =0.034), human rhinovirus (P=0.038), or human coronavirus NL63 (P=0.042). On the other hand, when human rhinovirus was coinfected with enterovirus (P=0.013) or parainfluenza 3 (P=0.019), the severity was increased. When human metapneumovirus coinfected with human bocavirus, the severity was increased (P=0.038).
REFERENCES
1. Roh EJ, Won YK, Lee MH, Chung EH. Clinical characteristics of patients with acute bronchiolitis who visited 146 Emergency Department in Korea in 2012. Allergy Asthma Respir Dis. 2015; 3:334–40.

2. Coastes BM. Wheezing in infants:bronchiolitis. Kliegman RM, Stanton BF, St. Geme JW Ш, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed.Philadelphia (PA): Elsevier;2016. p. 2044–8.
3. Bordley WC, Viswanathan M, King VJ, Sutton SF, Jackman AM, Sterling L, et al. Diagnosis and testing in bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2004; 158:119–26.
4. Skjerven HO, Megremis S, Papadopoulos NG, Mowinckel P, Carlsen KH, L⊘drup Carlsen KC, et al. Virus type and genomic load in acute bronchiolitis: severity and treatment response with inhaled adrenaline. J Infect Dis. 2016; 213:915–21.

5. Kang SY, Hong CR, Kang HM, Cho EY, Lee HJ, Choi EH, et al. Clinical and epidemiological characteristics of human metapneumovirus infections, in comparison with respiratory syncytial virus A and B. Pediatr Infect Vaccine. 2013; 20:168–77.

6. Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadom-ski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014; 134:e1474–502.

7. Park HJ, Kim JH, Chun YH, Lee SY, Kim SY, Kang JH. Clinical manifestations, management, and natural course of infants with recurrent bron-chiolitis or reactive airways disease. Pediatr Infect Vaccine. 2014; 21:37–42.

8. Colby TV. Bronchiolitis. Pathologic considerations. Am J Clin Pathol. 1998; 109:101–9.
9. Jeong Y, Hwang JH, Kwon JY, Shin J, Kwon JH, Han K, et al. Prediction of the severity and length of hospital stay in infants with acute bronchiolitis using the severity score. Allergy Asthma Respir Dis. 2016; 4:429–35.

10. Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997; 175:814–20.

11. Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012; 166:700–6.

12. Rhedin S, Lindstrand A, Rotzén-Östlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014; 133:e538–45.

13. Lim JS, Woo SI, Kwon HI, Baek YH, Choi YK, Hahn YS. Clinical characteristics of acute lower respiratory tract infections due to 13 respiratory viruses detected by multiplex PCR in children. Korean J Pediatr. 2010; 53:373–9.

14. Kim KH, Lee JH, Sun DS, Kim YB, Choi YJ, Park JS, et al. Detection and clinical manifestations of twelve respiratory viruses in hospitalized children with acute lower respiratory tract infections: focus on human metapneumovirus, human rhinovirus and human coronavirus. Korean J Pediatr. 2008; 51:834–41.
15. Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006; 43:585–92.

16. Huguenin A, Moutte L, Renois F, Leveque N, Talmud D, Abely M, et al. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012; 84:979–85.

17. Hasegawa K, Pate BM, Mansbach JM, Macias CG, Fisher ES, Piedra PA, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr. 2015; 15:77–81.

18. Rolfsjord LB, Skjerven HO, Carlsen KH, Mowinckel P, Bains KE, Bakke-heim E, et al. The severity of acute bronchiolitis in infants was associated with quality of life nine months later. Acta Paediatr. 2016; 105:834–41.

19. Mikalsen IB, Halvorsen T, Øymar K. The outcome after severe bronchiolitis is related to gender and virus. Pediatr Allergy Immunol. 2012; 23:391–8.

21. Rodriguez R, Ramilo O. Respiratory syncytial virus: how, why and what to do. J Infect. 2014; 68(Suppl 1):S115–8.

22. Boivin G, Caouette G, Frenette L, Carbonneau J, Ouakki M, De Serres G. Human respiratory syncytial virus and other viral infections in infants receiving palivizumab. J Clin Virol. 2008; 42:52–7.

23. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012; 6:71–7.

24. Park JS. Acute viral lower respiratory tract infections in children. Korean J Pediatr. 2009; 52:269–76.

25. Harada Y, Kinoshita F, Yoshida LM, Minh le N, Suzuki M, Morimoto K, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013; 32:441–5.

26. Rodríguez DA, Rodríguez-Martínez CE, Cárdenas AC, Quilaguy IE, Mayorga LY, Falla LM, et al. Predictors of severity and mortality in children hospitalized with respiratory syncytial virus infection in a tropical region. Pediatr Pulmonol. 2014; 49:269–76.

27. Asner SA, Rose W, Petrich A, Richardson S, Tran DJ. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect. 2015; 21:264.e1–6.

28. Brand HK, de Groot R, Galama J, Brouwer ML, Teuwen K, Hermans PW, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012; 47:393–400.

29. Calvo C, García-García ML, Pozo F, Paula G, Molinero M, Calderón A, et al. Respiratory syncytial virus coinfections with rhinovirus and human bocavirus in hospitalized children. Medicine (Baltimore). 2015; 94:e1788.

30. Mohapatra SS, Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev. 2008; 21:495–504.

31. Yun HJ. Respiratory syncytial virus infection and asthma. Pediatr Allergy Respir Dis. 1999; 9:24–31.
Fig. 1.
Year-round change of acute bronchiolitis with 16 respiratory viruses isolated for 768 hospitalized children.
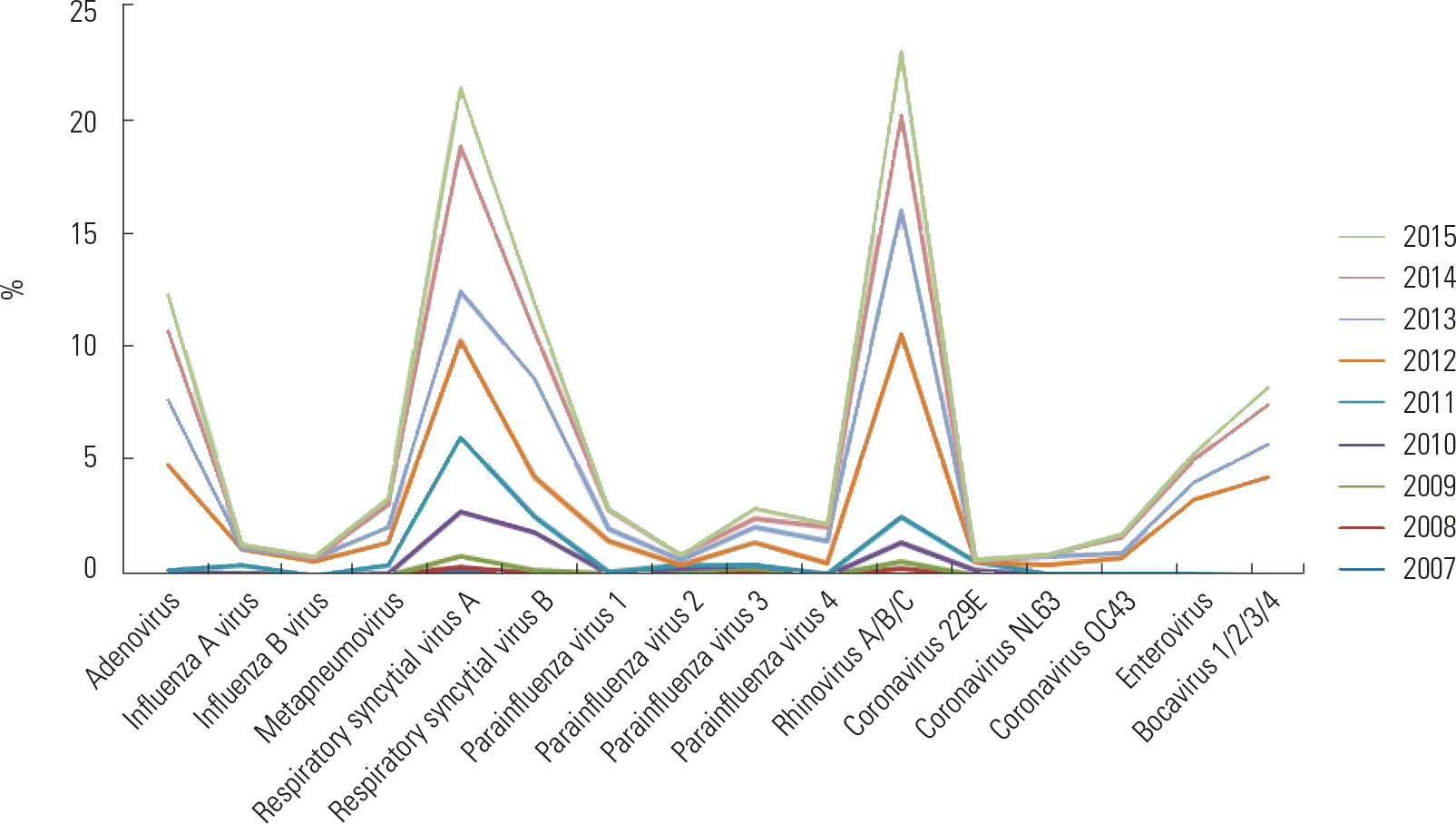
Table 1.
Clinical characteristics of hospitalized children with acute bronchiolitis
Table 2.
Clinical characteristics of hospitalized children with acute bronchiolitis analyzed according to disease severity
| Characteristic | Nonsevere (n=269) | Severe (n=499) | P-value |
|---|---|---|---|
| Age (mo)∗ | 3.39 | 2.62 | 0.054 |
| Hospitalization period (day)∗ | 4.2 | 6.35 | <0.001 |
| Fever (day)∗ | 2.20 | 1.60 | <0.001 |
| ICU hospitalization (day)∗ | 0 | 0.05 | 0.001 |
| CRP (mg/dL)∗ | 1.05 | 1.20 | 0.261 |
| Lymphocyte (%)∗ | 55.99 | 52.94 | 0.006 |
| Male sex† | 160 (59.5) | 321 (64.3) | 0.185 |
| Siblings, yes† | 151 (56.1) | 289 (57.9) | 0.634 |
| Previous wheezing episode† | 25 (9.3) | 83 (16.6) | 0.005 |
| Allergic disease family history† | 2 (0.7) | 8 (1.6) | 0.316 |
| Previous admission history† | 53 (19.7) | 145 (29.1) | 0.005 |
Table 3.
The ratio of single infection to coinfection of respiratory viruses isolated for 768 hospitalized children with acute bronchiolitis
Table 4.
Correlation of severity with coinfected viruses for 768 hospitalized children with acute bronchiolitis∗
| Infection |
Coinfected viruses |
||||||
|---|---|---|---|---|---|---|---|
| ADV (n=139) | hMPV (n=96) | RSV A (n=252) | RSV B (n=140) | hRV (n=257) | ETV (n=60) | hBoV (n=96) | |
| ADV | - | 0.239 (0.137) | –0.133 (0.034)† | 0.046 (0.589) | –0.002 (0.975) | 0.092 (0.484) | 0.012 (0.907) |
| Flu A | 0.103 (0.226) | - | 0.106 (0.094) | 0.053 (0.532) | 0.117 (0.061) | 0.066 (0.614) | 0.134 (0.195) |
| Flu B | –0.100 (0.239) | - | –0.074 (0.245) | - | –0.085 (0.175) | - | –0.106 (0.306) |
| hMPV | –0.004 (0.960) | - | 0.005 (0.940) | - | –0.081 (0.194) | 0.053 (0.688) | 0.092 (0.373) |
| RSV A | –0.051 (0.550) | 0.106 (0.516) | –0.057 (0.500) | –0.042 (0.503) | 0.073 (0.577) | –0.027 (0.794) | |
| RSV B | 0.115 (0.176) | - | –0.050 (0.432) | - | 0.077 (0.216) | –0.066 (0.615) | –0.069 (0.507) |
| hRV | 0.015 (0.863) | 0.189 (0.242) | –0.131 (0.038)† | 0.011 (0.897) | 0.195 (0.135) | 0.190 (0.064) | |
| PIV 1 | –0.098 (0.252) | - | –0.082 (0.197) | - | 0.055 (0.378) | 0.099 (0.452) | 0.085 (0.411) |
| PIV 2 | 0.127 (0.136) | - | –0.023 (0.718) | - | 0.093 (0.136) | 0.172 (0.189) | 0.160 (0.118) |
| PIV 3 | –0.070 (0.415) | 0.280 (0.080) | –0.023 (0.718) | - | 0.146 (0.019)‡ | 0.152 (0.246) | 0.053 (0.609) |
| PIV 4 | –0.057 (0.512) | - | 0.015 (0.840) | 0.068 (0.481) | 0.016 (0.809) | –0.029 (0.826) | –0.114 (0.268) |
| ETV | 0.132 (0.124) | 0.271 (0.115) | 0.121 (0.106) | –0.010 (0.920) | 0.164 (0.013)‡ | - | 0.170 (0.097) |
| hCoV 229E | - | - | –0.031 (0.627) | - | –0.031 (0.624) | –0.149 (0.257) | - |
| hCoV NL63 | –0.144 (0.093) | –0.136 (0.437) | –0.152 (0.042)† | - | –0.002 (0.981) | - | - |
| hCoV OC43 | –0.047 (0.587) | - | –0.024 (0.700) | –0.039 (0.651) | 0.062 (0.322) | - | –0.106 (0.306) |
| hBoV | –0.073 (0.397) | 0.351 (0.038)‡ | –0.034 (0.651) | 0.078 (0.418) | 0.063 (0.344) | 0.035 (0.792) | - |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download