Abstract
There have been few cases of albumin hypersensitivity reported, and there is limited information on this condition. When a patient is anaphylactic to a certain drug and no alternative drug is available to treat the underlying condition, desensitization is a reasonable option and can be performed successfully to treat the patient. A standard 12-step, 3-solution rapid desensitization protocol allows the safe readministration of a medication after certain types of immediate hypersensitivity. However, we demonstrated that a new 10-step, 1-solution desensitization protocol using antihistamine and leukotriene receptor antagonist as premedications, which was effective and safe in a patient with hypersensitivity. We report a 13-year-old boy with Gorham-stout syndrome who was presented with newly acquired albumin anaphylaxis and successfully treated with the 10-step rapid drug desensitization protocol.
Figures and Tables
Fig. 1
Serial X-ray images show the gradual increase in the pleural fluid of both lung fields in 2010 (A), in 2012 (B), in February 2015 (C), and in December 2015 (D).
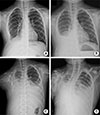
Fig. 2
Hypotension and desaturation occurred after albumin injections, however, vanished after desensitization. LTRA, Leukotriene receptor antagonist. ♦ indicates symptoms of flushing or rash.

Table 1
Rapid desensitization protocol which was applied to this case
References
1. Yoon JY, Lee JE, Park SW, Kang MJ, Lee YA, Yang SW, et al. Pamidronate treatment in 9-year-old boy diagnosed with gorham disease. J Korean Soc Pediatr Endocrinol. 2011; 16:189–192.

2. U.S.Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Ver. 4.0 [Internet]. Bethesda: National Cancer Institute;cited 2016 Aug 5. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf.
3. Thong BY. Clinical applications of drug desensitization in the Asia-Pacific region. Asia Pac Allergy. 2011; 1:2–11.

4. Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone. 2014; 63:47–52.

5. Gruchalla RS. Acute drug desensitization. Clin Exp Allergy. 1998; 28:Suppl 4. 63–64.
6. Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001; 35:1458–1464.

7. Li Q, Cohn D, Waller A, Backes F, Copeland L, Fowler J, et al. Outpatient rapid 4-step desensitization for gynecologic oncology patients with mild to low-risk, moderate hypersensitivity reactions to carboplatin/cisplatin. Gynecol Oncol. 2014; 135:90–94.

8. Ataca P, Atilla E, Kendir R, Bavbek S, Ozcan M. Successful desensitization of a patient with rituximab hypersensitivity. Case Reports Immunol. 2015; 2015:524507.

9. Won HK, Moon SD, Shim JS, Chung SJ, Kim GW, Kim SJ, et al. Successful rapid desensitization for cetuximab-induced anaphylaxis. Allergy Asthma Respir Dis. 2015; 3:294–296.

10. Liu A, Fanning L, Chong H, Fernandez J, Sloane D, Sancho-Serra M, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy. 2011; 41:1679–1689.

11. Hendrickson JE, Roubinian NH, Chowdhury D, Brambilla D, Murphy EL, Wu Y, et al. Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication. Transfusion. 2016; 56:2587–2596.

13. Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment [Internet]. Uppsala (Sweden): Uppsala Monitoring Centre;cited 2016 Aug 5. Available from: http://who-umc.org/Graphics/24734.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download