1. Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011; 22:155–160. PMID:
21332796.

2. Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Gillman MW, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014; 134:753–755. PMID:
25086866.

3. Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017; 140:145–153.e8. PMID:
28514997.

4. Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389:276–286. PMID:
27939035.

5. Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016; 316:1181–1192. PMID:
27654604.
6. Togias A, Cooper SF, Acebal ML, Assa'ad A, Baker JR Jr, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored Expert Panel. J Allergy Clin Immunol. 2017; 139:29–44. PMID:
28065278.

7. Netting MJ, Campbell DE, Koplin JJ, Beck KM, McWilliam V, Dharmage SC, et al. An Australian consensus on infant feeding guidelines to prevent food allergy: outcomes from the Australian Infant Feeding Summit. J Allergy Clin Immunol Pract. 2017; 5:1617–1624. PMID:
28499774.

8. Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. J Allergy Clin Immunol. 2015; 136:258–261. PMID:
26100082.

9. Lao-araya M, Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr Int. 2012; 54:238–243. PMID:
22168484.

10. Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol. 2011; 22:715–719. PMID:
21539613.

11. Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow's milk allergy in European children--EuroPrevall birth cohort. Allergy. 2015; 70:963–972. PMID:
25864712.
12. Xepapadaki P, Fiocchi A, Grabenhenrich L, Roberts G, Grimshaw KE, Fiandor A, et al. Incidence and natural history of hen's egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy. 2016; 71:350–357. PMID:
26514330.

13. Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JK, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013; 6:21. PMID:
24304599.

14. Akiyama H, Imai T, Ebisawa M. Japan food allergen labeling regulation--history and evaluation. Adv Food Nutr Res. 2011; 62:139–171. PMID:
21504823.

15. Kim M, Lee JY, Jeon HY, Yang HK, Lee KJ, Han Y, et al. Prevalence of immediate-type food allergy in Korean schoolchildren in 2015: a nationwide, population-based study. Allergy Asthma Immunol Res. 2017; 9:410–416. PMID:
28677354.

16. Jeong K, Kim J, Ahn K, Lee SY, Min TK, Pyun BY, et al. Age-based causes and clinical characteristics of immediate-type food allergy in Korean children. Allergy Asthma Immunol Res. 2017; 9:423–430. PMID:
28677356.

17. Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013; 3:3–14. PMID:
23403837.

18. Gray CL, Levin ME, du Toit G. Ethnic differences in peanut allergy patterns in South African children with atopic dermatitis. Pediatr Allergy Immunol. 2015; 26:721–730. PMID:
26267015.

19. Mahdavinia M, Fox SR, Smith BM, James C, Palmisano EL, Mohammed A, et al. Racial differences in food allergy phenotype and health care utilization among US children. J Allergy Clin Immunol Pract. 2017; 5:352–357.e1. PMID:
27888035.

20. Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008; 122:984–991. PMID:
19000582.

21. Koplin JJ, Peters RL, Ponsonby AL, Gurrin LC, Hill D, Tang ML, et al. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014; 69:1639–1647. PMID:
25041549.

22. Panjari M, Koplin JJ, Dharmage SC, Peters RL, Gurrin LC, Sawyer SM, et al. Nut allergy prevalence and differences between Asian-born children and Australian-born children of Asian descent: a state-wide survey of children at primary school entry in Victoria, Australia. Clin Exp Allergy. 2016; 46:602–609. PMID:
26728850.

23. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010; 126:324–331. 331.e1–331.e7. PMID:
20624649.

24. Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clin Exp Allergy. 2013; 43:642–651. PMID:
23711126.

25. Vereda A, van Hage M, Ahlstedt S, Ibañez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011; 127:603–607. PMID:
21093026.

26. Datema MR, Zuidmeer-Jongejan L, Asero R, Barreales L, Belohlavkova S, de Blay F, et al. Hazelnut allergy across Europe dissected molecularly: a EuroPrevall outpatient clinic survey. J Allergy Clin Immunol. 2015; 136:382–391. PMID:
25772593.
27. Le TM, Bublin M, Breiteneder H, Fernández-Rivas M, Asero R, Ballmer-Weber B, et al. Kiwifruit allergy across Europe: clinical manifestation and IgE recognition patterns to kiwifruit allergens. J Allergy Clin Immunol. 2013; 131:164–171. PMID:
23141741.

28. Wong L, Huang CH, Lee BW. Shellfish and house dust mite allergies: is the link tropomyosin. Allergy Asthma Immunol Res. 2016; 8:101–106. PMID:
26739402.

29. Gámez C, Zafra M, Boquete M, Sanz V, Mazzeo C, Ibáñez MD, et al. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res. 2014; 58:1915–1925. PMID:
24978201.

30. Thalayasingam M, Gerez IF, Yap GC, Llanora GV, Chia IP, Chua L, et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin Exp Allergy. 2015; 45:687–697. PMID:
25257922.

31. Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003; 33:956–961. PMID:
12859453.

32. Howell WM, Turner SJ, O'B Hourihane J, Dean TP, Warner JO. HLA class II DRB1, DQB1 and DPB1 genotypic associations with peanut allergy: evidence from a family-based and case-control study. Clin Exp Allergy. 1998; 28:156–162. PMID:
9515587.

33. Hong X, Hao K, Ladd-Acosta C, Hansen KD, Tsai HJ, Liu X, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun. 2015; 6:6304. PMID:
25710614.

34. Chen H, Common JE, Haines RL, Balakrishnan A, Brown SJ, Goh CS, et al. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol. 2011; 165:106–114. PMID:
21428977.

35. Osawa R, Konno S, Akiyama M, Nemoto-Hasebe I, Nomura T, Nomura Y, et al. Japanese-specific filaggrin gene mutations in Japanese patients suffering from atopic eczema and asthma. J Invest Dermatol. 2010; 130:2834–2836. PMID:
20686498.

36. Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011; 127:661–667. PMID:
21377035.

37. Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014; 134:867–875.e1. PMID:
25282568.

38. Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015; 135:164–170. PMID:
25457149.

39. Nomura T, Tsuge I, Inuo C, Nakajima Y, Kondo Y, Sugiura S, et al. Food sensitization in Japanese infants is associated with a common Filaggrin variant. Ann Allergy Asthma Immunol. 2013; 110:388–390.e1. PMID:
23622014.

40. Tan HT, Ellis JA, Koplin JJ, Matheson MC, Gurrin LC, Lowe AJ, et al. Filaggrin loss-of-function mutations do not predict food allergy over and above the risk of food sensitization among infants. J Allergy Clin Immunol. 2012; 130:1211–1213.e3. PMID:
22964107.

41. Leung DY. Atopic dermatitis: age and race do matter! J Allergy Clin Immunol. 2015; 136:1265–1267. PMID:
26549637.

42. Ashley SE, Tan HT, Peters R, Allen KJ, Vuillermin P, Dharmage SC, et al. Genetic variation at the Th2 immune gene IL13 is associated with IgE-mediated paediatric food allergy. Clin Exp Allergy. 2017; 47:1032–1037. PMID:
28544327.
43. Ashley SE, Tan HT, Vuillermin P, Dharmage SC, Tang ML, Koplin J, et al. The skin barrier function gene SPINK5 is associated with challenge-proven IgE-mediated food allergy in infants. Allergy. 2017; 72:1356–1364. PMID:
28213955.
44. Camargo CA Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007; 120:131–136. PMID:
17559916.

45. Mullins RJ, Clark S, Camargo CA Jr. Regional variation in epinephrine autoinjector prescriptions in Australia: more evidence for the vitamin D-anaphylaxis hypothesis. Ann Allergy Asthma Immunol. 2009; 103:488–495. PMID:
20084842.

46. Keet CA, Matsui EC, Savage JH, Neuman-Sunshine DL, Skripak J, Peng RD, et al. Potential mechanisms for the association between fall birth and food allergy. Allergy. 2012; 67:775–782. PMID:
22515802.

47. Thysen AH, Rasmussen MA, Kreiner-Møller E, Larsen JM, Følsgaard NV, Bønnelykke K, et al. Season of birth shapes neonatal immune function. J Allergy Clin Immunol. 2016; 137:1238–1246.e13. PMID:
26581916.

48. Kusunoki T, Morimoto T, Sakuma M, Mukaida K, Yasumi T, Nishikomori R, et al. Effect of eczema on the association between season of birth and food allergy in Japanese children. Pediatr Int. 2013; 55:7–10. PMID:
22978473.

49. Willits EK, Wang Z, Jin J, Patel B, Motosue M, Bhagia A, et al. Vitamin D and food allergies in children: a systematic review and meta-analysis. Allergy Asthma Proc. 2017; 38:21–28.

50. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369:1991–2000. PMID:
24256378.

51. Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013; 131:1109–1116. 1116.e1–1116.e6. PMID:
23453797.

52. Koplin JJ, Suaini NH, Vuillermin P, Ellis JA, Panjari M, Ponsonby AL, et al. Polymorphisms affecting vitamin D-binding protein modify the relationship between serum vitamin D (25[OH]D3) and food allergy. J Allergy Clin Immunol. 2016; 137:500–506.e4. PMID:
26260969.

53. Kim J, Lee JY, Han Y, Ahn K. Significance of Ara h 2 in clinical reactivity and effect of cooking methods on allergenicity. Ann Allergy Asthma Immunol. 2013; 110:34–38. PMID:
23244656.

54. Kim J, Lee J, Seo WH, Han Y, Ahn K, Lee SI. Changes in major peanut allergens under different ph conditions. Allergy Asthma Immunol Res. 2012; 4:157–160. PMID:
22548209.

55. World Health Organization. UNICEF. Global strategy for infant and young child feeding. Geneva: World Health Organization;2003.
56. American Academy of Pediatrics, Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000; 106:346–349. PMID:
10920165.
57. Boyce JA, Assa'a A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. Nutrition. 2011; 27:253–267. PMID:
21215925.

58. Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow's milk protein is protective against IgE-mediated cow's milk protein allergy. J Allergy Clin Immunol. 2010; 126:77–82.e1. PMID:
20541249.

59. Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010; 126:807–813. PMID:
20920771.
60. Tham EH, Lee BW, Chan YH, Loo EX, Toh JY, Goh A, et al. Low food allergy prevalence despite delayed introduction of allergenic foods-data from the GUSTO cohort. J Allergy Clin Immunol Pract. 2017; Forthcoming.

61. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372:803–813. PMID:
25705822.

62. Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389:276–286. PMID:
27939035.

63. Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016; 374:1733–1743. PMID:
26943128.

64. Klemans RJ, van Os-Medendorp H, Blankestijn M, Bruijnzeel-Koomen CA, Knol EF, Knulst AC. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy. 2015; 45:720–730. PMID:
25226880.

65. Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009; 124:771–778.e5. PMID:
19665774.

66. Hansen KS, Ballmer-Weber BK, Sastre J, Lidholm J, Andersson K, Oberhofer H, et al. Component-resolved
in vitro diagnosis of hazelnut allergy in Europe. J Allergy Clin Immunol. 2009; 123:1134–1141. 1141.e1–1141.e3. PMID:
19344939.
67. Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013; 132:393–399. PMID:
23582909.

68. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–896. PMID:
11344358.

69. Osterballe M, Bindslev-Jensen C. Threshold levels in food challenge and specific IgE in patients with egg allergy: is there a relationship? J Allergy Clin Immunol. 2003; 112:196–201. PMID:
12847499.

70. Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007; 119:1272–1274. PMID:
17337292.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


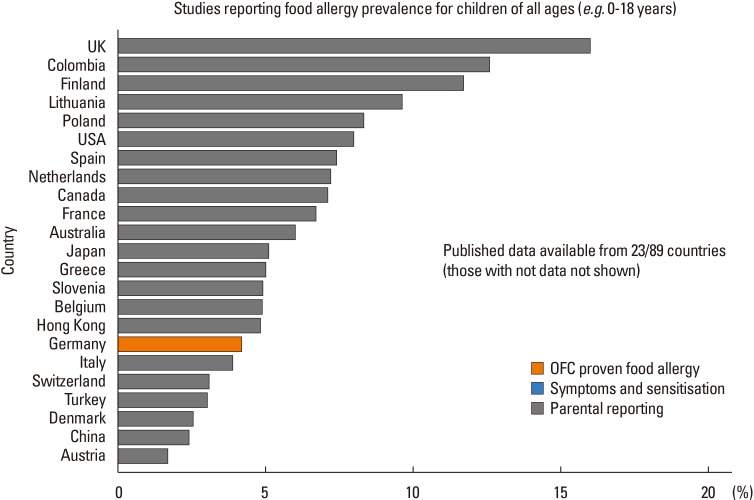
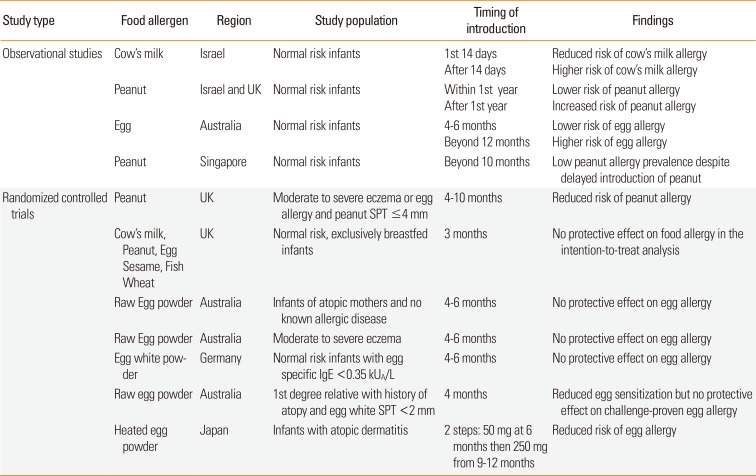
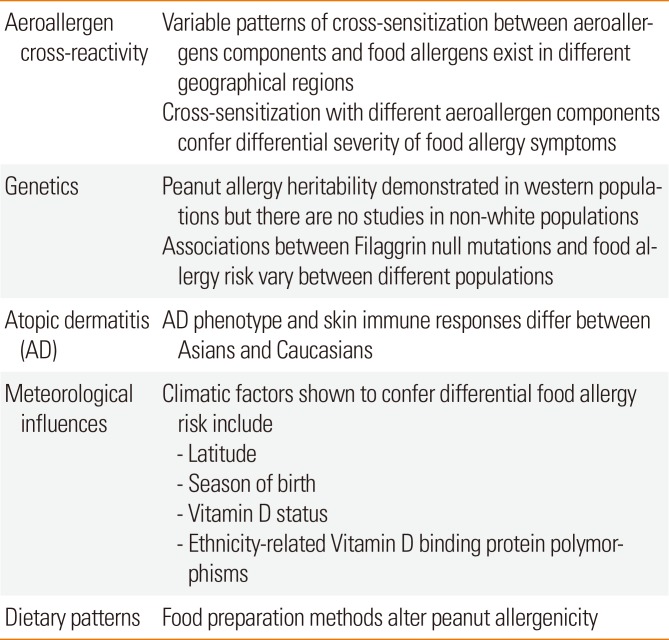
 XML Download
XML Download