Abstract
Purpose
Although the role of eosinophils in eosinophilic gastroenteritis (EGE) is not fully understood, they are believed to be a principal effector cell. Previous studies have demonstrated that eotaxin and its specific receptor, cysteine-cysteine chemokine receptor-3 (CCR3), play a central role in eosinophil trafficking into the gastrointestinal (GI) tract. Thus, we examined the targeting of CCR3 as a potential therapeutic intervention for EGE in a mouse model.
Methods
Eight- to 10-week-old BALB/c mice were intraperitoneally sensitized and intragastrically challenged with ovalbumin (OVA). Different groups of mice were administered either an anti-CCR3 antibody or a control IgG by intraperitoneal injection 1 hour before each OVA challenge. Eosinophilic inflammation in the intestinal mucosa, mucosal injury, and severity of diarrhea were compared between different groups at 1 hour after final OVA challenge.
Results
Anti-CCR3 antibody reduced the number of eosinophils in peripheral blood and intestinal mucosa, but not in bone marrow. This reduction was associated with restoration of reduced villous crypt ratio, increased intestinal epithelial cell proliferation, and weight loss induced by OVA challenge. However, Anti-CCR3 antibody had no effect on the level of OVA specific immunoglobulin E (IgE) and the expression of critical chemokines or cytokines in eosinophil trafficking into the GI tract, such as eotaxin-1, interleukin (IL)-5, and IL-13.
Eosinophilic gastroenteritis (EGE) is a member of eosinophilic gastrointestinal diseases (EGIDs) which include eosinophilic esophagitis, eosinophilic gastritis, EGE, and eosinophilic colitis. EGE is characterized by accumulation of eosinophils within the gastrointestinal (GI) tract. Various GI symptoms occur depending on the organ affected and the intensity of eosinophilic inflammation. Common symptoms are vomiting, irritability, abdominal pain, dysphagia, diarrhea, and weight loss.1 Recent electronic survey results indicate that the estimated incidence of EGE remains relatively low, with 22-28 cases per 100,000 patients.2 However, estimation of the actual incidence of EGE is difficult because many cases are not properly diagnosed or reported. Although the underlying etiology and pathophysiology of EGE are currently unknown, growing evidence suggests that atopy and food allergy appear to play a role.34
With the exception of the esophagus, eosinophils are a constitutive component of the GI tract, in which eosinophils play vital roles in host defense against parasitic infections and in normal tissue homeostasis. However, eosinophils are also implicated in the pathogenesis of various diseases. Although the role of eosinophils in EGE is not fully understood, they are believed to be a principal effector cell that induces GI tissue injury and disease pathogenesis.35
Of the mediators involved in trafficking of eosinophils into the GI tract, interleukin (IL)-5 and eotaxin play a central role.678 Previous studies have identified IL-5 as an essential eosinophil growth factor and eotaxin as a critical chemokine in eosinophil homing and tissue recruitment. Eotaxin-3 is a key player in the eosinophilia observed in eosinophilic esophagitis, while eotaxin-1 is involved in the lower GI eosinophilic diseases.9 Eosinophils express cysteine-cysteine chemokine receptor-3 (CCR3), a specific receptor for eotaxin, which is involved in selective recruitment of this leukocyte to sites of inflammation. Therefore, eotaxin and its receptor, CCR3, are potential therapeutic targets for EGE.
In this study, we used a mouse model of oral food allergen-induced GI eosinophilic inflammation to determine whether targeting of CCR3 is an effective therapeutic intervention in EGE.
In this study, 8- to 10-week-old BALB/c mice (8 mice/group; Orient Bio Inc., Seongnam, Korea) were sensitized and challenged with ovalbumin (OVA; grade V; Sigma-Aldrich, St. Louis, MO, USA), as previously described.510 In brief, mice were intraperitoneally sensitized on days 0 and 14 (50 µg of OVA adsorbed to 1 mg of aluminum hydroxide [Sigma-Aldrich] adjuvant) and intragastrically challenged on days 28, 30, 32, 35, 37, and 39 (50 mg of OVA suspended in 250 µL of phosphate buffered saline [PBS]) (Fig. 1). Control age- and sex-matched BALB/c mice were sensitized and challenged with PBS. Mice were sacrificed 1 hour after the final OVA challenge on day 39. Mice jejunums were removed 10 cm distal to the stomach, fixed with 4% paraformaldehyde solution for 24 hours, and embedded in paraffin, and 5-µm tissue sections were prepared for analysis. Stained and immunostained slides were quantified by an image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MA, USA) under fixed light microscope conditions. An investigator blinded to the study group performed slide analysis. All animal experimental protocols were approved by the Korea University Animal Subjects Committee.
Different groups of mice were administered 5 mg/kg of either a rat anti-mouse CCR3 mAb (kindly provided by James Lee, PhD, Mayo Clinic, Scottsdale, AZ, USA) or a control rat IgG (R&D Systems, Minneapolis, MN, USA) in 100 µL PBS via intraperitoneal injection 1 hour before each OVA challenge, as previously described (Fig. 1).11
Peripheral blood (PB) was collected from different groups of mice by cardiac puncture, as previously described.12 Erythrocytes were lysed using a 1:10 solution of 100 mM potassium carbonate-1.5 M ammonium chloride. The remaining cells were re-suspended in 1 mL of PBS. Bone marrow (BM) cells were flushed from femurs with 1 mL of PBS, centrifuged, and re-suspended in 1 mL of PBS. To perform differential cell counts, 200 µL of PB cell suspension or 20 µL of bone marrow cell suspension were cytospun onto microscope slides and air-dried. Slides were stained with Wright-Giemsa, and differential cell counts were performed under a light microscope.
Eosinophils in jejunal tissue were detected by immunohistochemistry using an anti-mouse major basic protein (MBP) antibody (kindly provided by James Lee, PhD, Mayo Clinic), as previously described.13 Tissue sections were also stained for mucosal mast cells using chloroacetate esterase (Sigma-Aldrich) activity, as previously described,14 and then lightly counterstained with hematoxylin. Quantification of eosinophils and mast cells was performed using a light microscope attached to an image analysis system. Results are expressed as the number of eosinophils or mast cells per mm2 of lamina propria. At least 10 randomly selected areas of jejunal mucosa were counted in each slide at ×20 magnification.
To investigate intestinal epithelial cell proliferation, 5′-bromodeoxyuridine (5′-BrdU) (Zymed Laboratories Inc., South San Francisco, CA, USA) incorporation of jejunal epithelial cells was measured as previously described.15 Briefly, mice were intraperitoneally injected with 0.25 mL of 5′-BrdU 3 hours before sacrifice. Then, immunohistochemical detection of BrdU in intestinal mucosal epithelial cells was performed using a BrdU Immunohistochemistry Kit (Chemicon, Temecula, CA, USA) according to the manufacturer's instructions. Epithelial cell proliferation was quantitated as the percentage of BrdU-labeled cells per 500 crypt cells assessed in well-oriented crypt-villous units.
Intestinal eosinophilic inflammation is associated with decreased villous height, increased crypt depth, and reduced villus crypt ratio. To investigate intestinal villus/crypt morphologic changes, villous height and crypt depth in the jejunum were measured using a light microscope attached to an image analysis system. Ten well-oriented crypt-villus units from hematoxylin-eosin-stained sections were randomly chosen for villous height and crypt depth measurement at ×10 magnification. Results are expressed as villus/crypt ratio.
Intestinal tissue was homogenized in 2.0 mL of PBS (pH 7.4), and supernatants were obtained by centrifugation (1,800 rpm for 10 minutes) and frozen at -80℃ in polypropylene tubes until assayed. Eotaxin-1, IL-5, and IL-13 levels in intestinal mucosa were measured by enzyme-linked immunosorbent assay (ELISA) using immunoassay kits (R&D systems) according to the manufacturer's instructions. Intestinal tissue protein levels were quantitated using a BCA protein assay (Pierce, Rockford, IL, USA). Results are expressed as pg eotaxin-1 or IL-5/mg intestinal mucosa protein.
Serum OVA-specific IgE concentrations were determined by ELISA. Ninety-six well plates were pre-coated with 100 µg/mL OVA, blocked with 10% FBS. Mouse serum samples diluted 1:50 were added to the OVA-coated wells. Two hours after incubation at room temperature, the plates were washed with wash buffer and biotinylated anti-mouse IgE (BD Bioscience, San Diego, CA, USA) was added. The optical density (OD) was read at 450 nm within 30 minutes.
Diarrhea is difficult to quantitate in mice, so differences in weight gain were measured as a surrogate end-point for the severity of diarrhea, as previously described.5 Mice were weighed in grams using PAG 2102 (OHAUS Corporation, Parsippany, NJ, USA) on days 0 and 39. The results are expressed as % weight gain from day 0.
The number of PB eosinophils significantly increased in OVA-challenged mice compared with non-OVA-challenged ones (9.75%±2.96% vs 2.17%±0.82% eosinophils, P=0.016). Administration of anti-CCR3 antibody significantly reduced OVA-induced PB eosinophilia compared with that of control antibody (1.63%±0.43% vs 9.75%±2.96% eosinophils, P=0.006; Fig. 2A).
The number of BM eosinophils also significantly increased in the OVA-challenged mice compared with the non-OVA-challenged ones (10.78%±1.39% vs 6.13%±0.63% eosinophils, P=0.016). In contrast to the significant reduction in PB eosinophils, administration of anti-CCR3 antibody had no effect on the number of BM eosinophils (Fig. 2B).
The number of eosinophils in the intestinal mucosa significantly increased in OVA-challenged mice compared with non-OVA-challenged ones (358.20±12.40 vs 179.90±8.12 eosinophils/mm2, P<0.001). Administration of anti-CCR3 antibody almost completely inhibited OVA-induced eosinophil recruitment into the intestinal mucosa compared with that of control antibody (10.37±2.04 vs 358.20±12.40 eosinophil/mm2, P<0.001; Fig. 3C).
The number of mast cells in the intestinal mucosa also significantly increased in OVA-challenged mice compared with non-OVA-challenged mice (14.49±1.54 vs 134.90±10.23 mast cells/mm2, P<0.001). In contrast to the significant reduction in the number of eosinophils, the administration of anti-CCR3 antibody had no effect on the number of mast cells in intestinal mucosa (Fig. 3D).
Because eotaxin-1, IL-5, and IL-13 are important in eosinophil trafficking, the effect of anti-CCR3 antibody administration on the expression of these chemokine and cytokines in the intestinal mucosa was assessed. The expression of these mediators significantly increased in OVA-challenged mice compared with non-OVA-challenged ones. However, administration of anti-CCR3 antibody had no effect on the expression level of these mediators (Fig. 4A-C).
OVA-specific IgE was significantly increased in OVA-challenged mice compared with non-OVA-challenged ones (0.69±0.11 vs 0.25±0.07, P<0.007). Administration of an anti-CCR3 antibody had no effect on the increased level of OVA-specific IgE (Fig. 4D).
The percentage of BrdU-labeled jejunal epithelial cells significantly increased in OVA-challenged mice compared with non-OVA-challenged ones (69.03%±6.54% vs 20.83%±3.05% BrdU-labeled intestinal epithelial cells, P<0.001). Administration of anti-CCR3 antibody significantly reduced OVA-induced intestinal epithelial cell proliferation compared with administration of control antibody (43.88%±7.93% vs 69.03%±6.54% BrdU-labeled intestinal epithelial cells, P=0.030; Fig. 5).
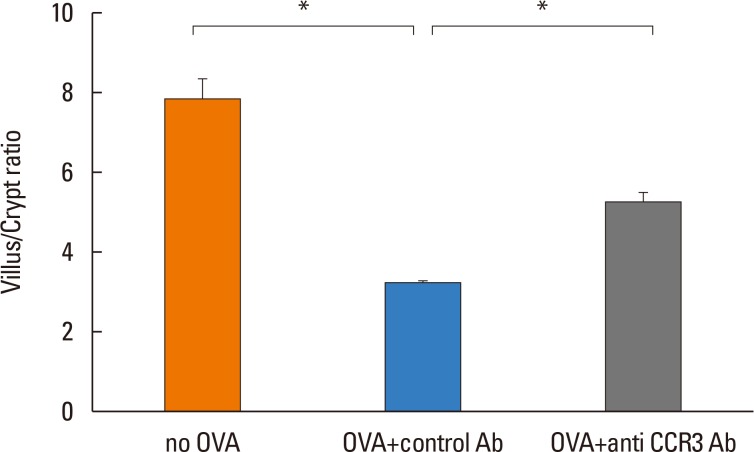
The villus/crypt ratio of intestinal mucosa was significantly reduced in OVA-challenged mice compared with non-OVA-challenged ones (3.14±0.16 vs. 7.79±0.61, P<0.001). Administration of anti-CCR3 antibody significantly restored the OVA-induced reduction in the villus/crypt ratio compared with administration of control antibody (5.24±0.28 vs 3.14±0.16, P<0.001; Fig. 6).
Baseline body weight at day 0 was not different between the experimental groups. Weight gain was significantly reduced in OVA-challenged mice compared with non-OVA-challenged ones (-8.74%±0.84% vs 4.87%±1.09% weight gain, P<0.001). Administration of anti-CCR3 antibody partially restored the OVA-induced weight loss compared with administration of control antibody (-4.18%±1.44% vs -8.74%±0.84% weight gain, P=0.013).
In this study, we demonstrated that administration of an anti-CCR3 antibody significantly reduced eosinophilic inflammation in the intestinal mucosa. This was associated with significantly reduced intestinal epithelial cell proliferation, normalization of intestinal villous/crypt ratio, and reduction of weight loss induced by diarrhea. Although previous studies have demonstrated that eotaxin and its specific receptor, CCR3, play pivotal roles in eosinophil trafficking into the GI tract at baseline,7 this is the first study to demonstrate that targeting of CCR3 can affect eosinophil level in food allergen-induced GI eosinophilic inflammation.
Elimination diets and systemic or topical corticosteroids have been used to treat EGE. However, a standard therapy has not yet been defined. Elimination diets implicated by skin tests have shown variable effects, but amino acid-based elemental diets resolved both symptoms and tissue eosinophilia in a large portion of EGE patients.16 The main drawbacks of dietary treatment are symptom recurrence with reintroduction of non-elemental foods, risk of nutritional imbalance, and psychological burden on patients and their families. Systemic or topical corticosteroids have showed favorable results in EGE patients in which dietary treatment is not feasible or effective.1718 However, long-term use of systemic corticosteroids is limited due to the risk of systemic side effects, and the role of topical corticosteroids as a long-term treatment strategy is still unclear. Furthermore, many EGE patients do not achieve complete symptom remission with these approaches. Therefore, there is still a great need for new therapeutic interventions.
Previous studies have suggested that eosinophils play a key role in tissue injury and GI symptoms associated with EGIDs.51519 CCR3 is expressed on the cell surface of eosinophils and can bind several eosinophil-active chemokines such as eotaxin, RANTE, and MCP-3. Among these chemokines, eotaxin is the most potent chemoattractant for eosinophils and exclusive ligands for CCR3, making CCR3 an attractive therapeutic target in eosinophil-mediated diseases. There have been a variety of approaches for targeting CCR3 using small molecule receptor antagonists, monoclonal antibodies, and antisense oligonucleotides. Although a few molecules have advanced into the clinic trials for patients with asthma and/or allergic rhinitis based on the results from animal models, early results were not promising.2021 However, human trials of monoclonal anti-CCR3 antibody have not been initiated. In addition, recent emergence of CCR3 as a novel target for eosinophilic esophagitis and age-related macular degeneration suggests that CCR3 efficacy may be different in various disease setting.20
In this study, the number of jejunal eosinophils significantly increased in OVA-challenged mice compared with non-OVA-challenged ones. Administration of anti-CCR3 antibody significantly reduced eosinophilic inflammation in the intestinal mucosa, tissue injury, and weight loss induced by diarrhea. However, administration of anti-CCR3 antibody had no effect on the number of BM eosinophils and the expression of eotaxin-1 and T helper 2 (Th2) cytokines involved in eosinophil trafficking into the GI tract, such as IL-5 and IL-13. In this regard, previous studies showed that mouse Th2 cells did not express detectable levels of CCR3 and treatment with anti-CCR3 antibody inhibited the migration and differentiation of bone marrow CD34+ progenitor cells, especially eosinophil-lineage progenitors.1122 These results suggest that anti-CCR3 antibody decreases tissue eosinophilic inflammation by reducing recruitment from BM into PB and sites of inflammation, rather than by decreasing BM production of eosinophils in mouse models. Our results are in consistent with those of previous studies.
In addition to the level of Th2 cytokines, anti-CCR3 antibody-treated mice also had showed similar levels of serum OVA-specific IgE with OVA-challenged ones. Because no CCR3 has been detected on B cells, the similar levels of OVA-specific IgE in mice treated with the anti-CCR3 antibody could be due to no effects of anti-CCR3 antibody on the increased levels of IL-13 which contribute to IgE synthesis.
While the inhibitory effect of the antibody on eosinophil recruitment into the GI mucosa was potent enough to ameliorate eosinophil number to below normal, the effect on EGE symptoms was modest in this study. This suggests that other cells are also involved. Mast cells play a critical role in the development of EGE symptoms, especially diarrhea.10 Because CCR3 is also expressed on mast cells, we examined the effect of anti-CCR3 antibody administration on the number of mast cells in intestinal mucosa. The number of mast cells in the GI mucosa significantly increased in OVA-challenged mice. However, in contrast to the effect of anti-CCR3 antibody on eosinophilic infiltration into the GI mucosa, the antibody did not affect accumulation of mast cells in the GI mucosa. In this regard, previous studies have suggested the different role of CCR3 on mast cells in humans and mice. In vitro studies using human mast cells have demonstrated migration to the CCR3 ligand eotaxin,23 whereas murine mast cells did not migrate toward CCR3 ligands.24 The number of intestinal mast cells in response to helminth infection and the number of basal mast cell progenitors were not significantly different between CCR3-/- and wild type mice.2526 Furthermore, eotaxin-1/CCR3 was required for IgE-mediated degranulation of murine mast cells.27 Our results are in agreement with those of previous studies. Therefore, it is possible that the effect of anti-CCR3 antibody on the severity of diarrhea could partially result from inhibition of mast cell activation.
In summary, this study showed that anti-CCR3 antibody could significantly reduce the severity of eosinophilic inflammation, mucosal injury, and weight loss associated with diarrhea in a mouse model of food allergen-induced GI eosinophilic inflammation. Thus, CCR3 may be a novel therapeutic target for the treatment of EGE and other GI eosinophil-mediated diseases.
ACKNOWLEDGMENTS
We would like to thank Dr. James Lee (Mayo Clinic, Scottsdale, AZ, USA) for his kind gift of anti-cysteine-cysteine chemokine receptor-3 (CCR3) mAb. This research was supported by a grant from the National Research Foundation of Korea (NRF-2009-0070179) and Korea University Grant as well as the ‘Program of Environmental Health Center for Asthma’ funded by the Ministry of Environment, Republic of Korea.
References
1. Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil-associated gastrointestinal disorders: a world-wide-web based registry. J Pediatr. 2002; 141:576–581. PMID: 12378201.

2. Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011; 52:300–306. PMID: 21057327.

3. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113:11–28. PMID: 14713902.

4. Chehade M, Aceves SS. Food allergy and eosinophilic esophagitis. Curr Opin Allergy Clin Immunol. 2010; 10:231–237. PMID: 20410819.

5. Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009; 131:157–169. PMID: 19135419.

6. Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994; 179:881–887. PMID: 7509365.

7. Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998; 95:6273–6278. PMID: 9600955.

8. Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev. 2001; 179:163–172. PMID: 11292020.

9. Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009; 101:81–121. PMID: 19231593.
10. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003; 112:1666–1677. PMID: 14660743.

11. Ben S, Li X, Xu F, Xu W, Li W, Wu Z, et al. Treatment with anti-CC chemokine receptor 3 monoclonal antibody or dexamethasone inhibits the migration and differentiation of bone marrow CD34 progenitor cells in an allergic mouse model. Allergy. 2008; 63:1164–1176. PMID: 18699933.
12. Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007; 109:4280–4287. PMID: 17272508.

13. Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004; 113:551–560. PMID: 14966564.

14. Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996; 135:279–290. PMID: 8858180.

15. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001; 107:83–90. PMID: 11134183.

16. Chehade M, Magid MS, Mofidi S, Nowak-Wegrzyn A, Sampson HA, Sicherer SH. Allergic eosinophilic gastroenteritis with protein-losing enteropathy: intestinal pathology, clinical course, and long-term follow-up. J Pediatr Gastroenterol Nutr. 2006; 42:516–521. PMID: 16707973.
17. Tan AC, Kruimel JW, Naber TH. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol. 2001; 13:425–427. PMID: 11338074.

18. Siewert E, Lammert F, Koppitz P, Schmidt T, Matern S. Eosinophilic gastroenteritis with severe protein-losing enteropathy: successful treatment with budesonide. Dig Liver Dis. 2006; 38:55–59. PMID: 16326154.

19. Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001; 2:353–360. PMID: 11276207.

20. Pease JE, Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin Drug Discov. 2014; 9:467–483. PMID: 24641500.

21. Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, Sousa AR, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014; 44:508–516. PMID: 24286456.

22. Grimaldi JC, Yu NX, Grunig G, Seymour BW, Cottrez F, Robinson DS, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3). J Leukoc Biol. 1999; 65:846–853. PMID: 10380909.

23. de Paulis A, Annunziato F, Di Gioia L, Romagnani S, Carfora M, Beltrame C, et al. Expression of the chemokine receptor CCR3 on human mast cells. Int Arch Allergy Immunol. 2001; 124:146–150. PMID: 11306952.

24. Collington SJ, Westwick J, Williams TJ, Weller CL. The function of CCR3 on mouse bone marrow-derived mast cells in vitro. Immunology. 2010; 129:115–124. PMID: 20050333.
25. Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, et al. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002; 168:5730–5736. PMID: 12023373.

26. Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, et al. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005; 105:4308–4313. PMID: 15705791.

27. Miyazaki D, Nakamura T, Ohbayashi M, Kuo CH, Komatsu N, Yakura K, et al. Ablation of type I hypersensitivity in experimental allergic conjunctivitis by eotaxin-1/CCR3 blockade. Int Immunol. 2009; 21:187–201. PMID: 19147836.

Fig. 1
Experimental protocol. Mice were intraperitoneally sensitized on days 0 and 14, and intragastrically challenged with OVA on days 28, 30, 32, 35, 37, and 39. One hour before each OVA challenge, anti-CCR3 or isotype control antibody was intraperitoneally administered (arrows). Mice were sacrificed 1 hour after the final OVA challenge, and their jejunums were analyzed. OVA, ovalbumin; CCR3, cysteine-cysteine chemokine receptor-3.
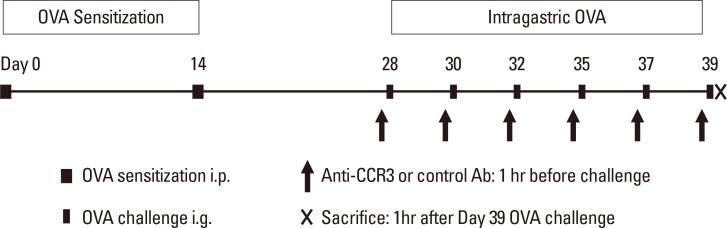
Fig. 2
Eosinophils in the bone marrow and PB. PB was collected by cardiac puncture and bone marrow cells were flushed from the femurs on day 39. Cell suspensions were cytospun onto microscope slides and then stained with Wright-Giemsa. Differential cell counts were performed in PB (A) and BM (B) cells. Data are expressed as means±SE. PB, peripheral blood; BM, bone marrow; SE, standard error. *P<0.05.
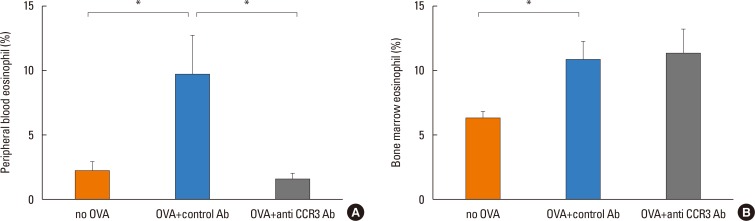
Fig. 3
Eosinophils and mast cells in the jejunum. Eosinophils and mast cells in the jejunum were detected using anti-MBP immunostain (A) and chloroacetate esterase staining (B), respectively. Red dots indicate MBP+ or chloroacetate esterase+ cells. The numbers of eosinophils (C) and mast cells (D) per mm2 of jejunal lamina propria were quantitated in different groups of mice. Data are expressed as means±SE. MBP, major basic protein; SE, standard error. *P<0.001.
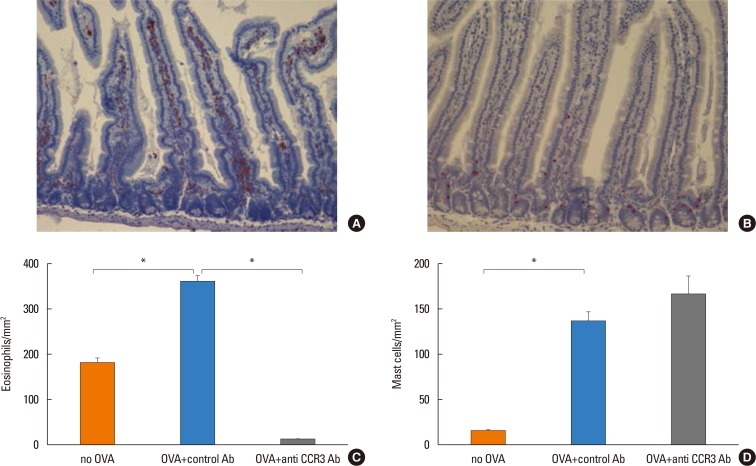
Fig. 4
Expression of eotaxin, IL-5, IL-13, and serum OVA-pecific IgE. Intestinal tissue was homogenized in 2.0 mL of PBS (pH 7.4) and supernatants assayed by ELISA for eotaxin-1 (A), IL-5 (B), and IL-13 (C). Intestinal tissue protein levels were quantitated using a BCA protein assay (Pierce, Rockford, IL, USA). Results are expressed as pg/mg intestinal mucosa protein. Serum OVA-specific IgE levels (D) were measured by ELISA on day 39 in different groups of mice. Data are expressed as means±SE. IL, interleukin; OVA, ovalbumin; IgE, immunoglobulin E; PBS, phosphate buffered saline; ELISA, enzyme-linked immunosorbent assay; SE, standard error. *P<0.05; †P<0.005.
Fig. 5
Mucosal epithelial cell proliferation in the jejunum. The incorporation of BrdU in the mucosal epithelial cells was measured 3 hours after intraperitoneal injection of 5′-BrdU on day 39. (A) BrdU-labeled epithelial cells (dark brown dots) in the jejunal mucosa. (B) The percentage of BrdU-labeled cells per 500 crypt cells was quantitated in different groups of mice. Data are expressed as means±SE. BrdU, bromodeoxyuridine; SE, standard error. *P<0.05; †P<0.001.
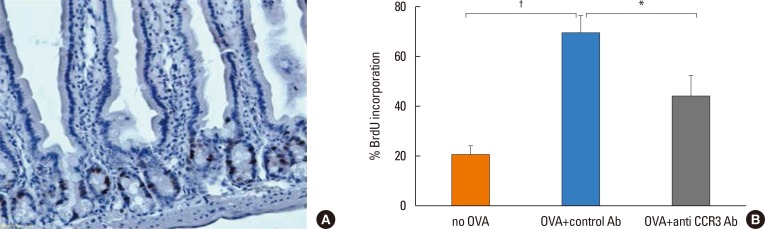
Fig. 6
Villus/crypt morphologic changes. Using a light microscope attached to an image-analysis system, villous height and crypt depth in jejunum were measured in ten randomly selected crypt-villous units from hematoxylin-eosin stained sections at ×10 magnification. Data are expressed as means±SE. SE, standard error. *P<0.001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download