Abstract
Levodropropizine is commonly used as an antitussive drug for acute and chronic cough. It is a non-opioid agent with peripheral antitussive action via the modulation of sensory neuropeptide levels in the airways. Thus, levodropropizine has a more tolerable profile than opioid antitussives. However, we experienced 3 cases of levodropropizine-induced anaphylaxis. Three patients commonly presented with generalized urticaria, dyspnea, and collapse after taking cold medication including levodropropizine. To find out the culprit drug, we performed skin tests, oral provocation tests (OPTs), and basophil activation tests (BATs). Two patients were confirmed as having levodropropizine-induced anaphylaxis by OPTs, and one of them showed positive to skin prick tests (SPTs). The other patient was confirmed by skin tests and BATs. When we analyzed pharmacovigilance data related to levodropropizine collected for 5 years, most cases (78.9%) had allergic reactions, such as rash, urticaria, angioedema, and anaphylaxis. Therefore, physicians should consider that levodropropizine can be a culprit drug, when anaphylaxis occurs after taking anti-cough or common cold medication.
Levodropropizine is commonly used as an antitussive drug for acute and chronic cough.1 It is a non-opioid agent with peripheral antitussive action via the modulation of sensory neuropeptide levels in the respiratory tract.2 Thus, levodropropizine has fewer sedative effects and a more tolerable profile than opioid antitussives. Common adverse effects of levodropropizine are somnolence, vomiting, epigastric pain, and diarrhea.3 Here, we report on 3 cases of levodropropizine-induced anaphylaxis and reviewed levodropropizine-induced adverse drug reactions (ADRs) using Korea's national wide pharmacovigilance data from January 2011 to June 2015.
The first case was a 67-year-old male referred to the emergency department due to anaphylaxis after taking cough suppressants, including acetaminophen, pseudoephedrine, theobromine, and levodropropizine. On arrival, he was unconscious and his blood pressure was 60/40 mmHg. He received emergency care for anaphylaxis and recovered. To identify the culprit drug, an allergic workup was performed in the outpatient clinic 2 weeks later. The results of skin prick tests (SPTs) using the drugs administered were negative, except for levodropropizine. SPTs performed using levodropropizine diluents induced a positive reaction (allergen/histamine [A/H] ratio of 3+ at 1 mg/mL). The second case was a 40-year-old female transferred to our hospital for the emergency management of anaphylaxis. She experienced loss of consciousness within 10 minutes after taking cold medications, including amoxicillin/clavulanic acid, mosapride, acetaminophen, and levodropropizine. She had had a similar experience 3 years earlier after taking cold medications. The results of serum-specific immunoglobulin E (IgE) to amoxicilloyl and SPTs using amoxicillin diluents were negative. Thus, we planned oral provocation tests (OPTs) to identify the culprit drugs in these 2 patients, and the results of OPTs using the administered drugs (acetaminophen, pseudoephedrine, and theobromine in patient 1; amoxicillin/clavulanic acid, mosapride, and acetaminophen in patient 2) were negative. However, after taking 30 mg of levodropropizine, both patients showed generalized urticaria, itching, and dyspnea within 30 minutes. The third case was a 22-year-old female who visited the outpatient clinic for a workup of past anaphylaxis events. When she was 10 years old, she experienced generalized urticaria, angioedema, mild dyspnea, and faintness 15-20 minutes after taking cold medications, including deoxyribonuclease, acetaminophen, cetirizine, pseudoephedrine, ambroxol, cefaclor, and levodropropizine. She experienced 2 similar events. Acetaminophen and levodropropizine were commonly included in the previous 3 events. We performed SPTs and intradermal tests (IDTs) with acetaminophen, levodropropizine, and cefaclor. The results of SPTs and IDTs for cefaclor and acetaminophen as well as specific IgE to cefaclor were all negative. However, the IDT using levodropropizine induced a positive response (A/H ratio of 3+ at 0.1 mg/mL). To confirm the diagnosis of anaphylaxis due to levodropropizine, we planned BATs instead of OPTs, according to the previously described method.4 We found a significant up-regulation of CD63 expression after stimulation with levodropropizine (Figure). The OPTs were negative for cefaclor and acetaminophen in this patient. Based on the SPT and BAT results, we confirmed levodropropizine-induced anaphylaxis.
Pharmacovigilance data about levodropropizine were collected from the Korea Institute of Drug Safety-Korea Adverse Event Reporting System (KIDS-KAERS) database of the Korea Institute of Drug Safety and Risk Management (Ministry of Food and Drug Safety) for 5 years (January 2011-June 2015). ADRs that were classified as certain or probable/likely were selected, and patients taking levodropropizine as a concomitant drug were excluded from the analysis. There were 162 ADR reports (from 102 persons) related to levodropropizine. A total of 38 reports (25 persons) considered having causal relationships were chosen for analysis (Table 1). The median age was 48.5 years (range 0–67 years), and 40.0% of the patients were male. The most common ADR was rash (26.3%), followed by urticaria (23.7%), angioedema (13.2%), rash acneform (7.9%), and anaphylaxis (5.3%). Non-immunologic reactions such as neuropathy, headache, and palmar-plantar erythrodysesthesia, were also reported.
Levodropropizine is a frequently used antitussive drug because it has fewer side effects than centrally acting opioid antitussives. However, we experienced 3 cases of anaphylaxis caused by levodropropizine.
Pharmacovigilance data related to levodropropizine showed that ADRs occurred in young age. Rash or urticaria developed in infants and a 7-year-old child. Most cases were immunological drug adverse reactions. Cutaneous manifestation was most common. IgE-mediated reactions, such as urticaria, angioedema, and anaphylaxis, accounted for 44.7% in all cases. The database was a self-reported system that may have underestimated the true incidence of ADRs caused by this drug. Although we could not estimate the incidence of levodropropizine-induced allergic reactions, these reactions may happen not infrequently and physicians pay attention to allergic reactions to levodropropizine in all ages.
The mechanism of levodropropizine-induced allergic reactions has not been fully evaluated; however, because it is a small molecule, it may be conjugated to a carrier protein, inducing an IgE immune response.5 Two of the cases described here showed positive SPT or IDT responses, suggesting IgE-mediated reactions. Moreover, in the third case, BATs showed significant up-regulation of the basophil activation marker, CD63 after stimulation with levodropropizine in a dose-dependent manner. In our cases, patients experienced moderate to severe degree of anaphylaxis caused by levodropropizine,6 and literature review shows additional 3 cases of anaphylaxis (Table 2).789 The mechanism of severe anaphylaxis development after levodropropizine ingestion has not been determined; however, an IgE-mediated reaction, as well as direct basophil or mast cell activation, may be involved.
Levodropropizine is frequently co-prescribed with NSAIDs or antibiotics in respiratory tract infections, as in our cases. NSAIDs and antibiotics are well-known drugs to cause allergic reactions commonly. Therefore, in previous cases,789 identification of the culprit drug was confirmed through open-label OPTs to exclude NSAIDs or antibiotics with having a risk of eliciting an anaphylaxis. However, in most reported cases, IDTs showed positive reactions, suggesting that skin tests could be a reliable method. Moreover, in our third case, we confirmed the identification of culprit drug using BATs without performing potentially dangerous drug challenge tests.
In conclusion, we reported on 3 cases of anaphylaxis due to levodropropizine, which were confirmed by SPTs, OPTs, and BATs. Clinicians should keep in mind that levodropropizine can be a culprit drug when anaphylaxis occurs after taking anti-cough or common cold medication.
Figures and Tables
Figure
Expression of CD63 on basophils induced by levodropropizine in the patient and controls. Controls consisted of 1 atopic and 1 non-atopic individuals. SI indicates stimulation index (percentage of basophils activated by the drug divided by the percentage of activated basophils in the negative controls). SI, stimulation index (percentage of basophils activated by the drug divided by the percentage of activated basophils in the negative controls).
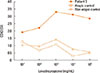
Table 1
Clinical manifestations of ADRs to levodropropizine in KIDS-KAERS

Table 2
Characteristics of reported cases of levodropropizine-induced anaphylaxis in Korea

ACKNOWLEDGMENTS
This research was supported by a grant from the Ministry of Food and Drug Safety to operation of the regional pharmacovigilance center in 2016.
References
1. De Blasio F, Virchow JC, Polverino M, Zanasi A, Behrakis PK, Kilinç G, et al. Cough management: a practical approach. Cough. 2011; 7:7.
2. Zanasi A, Lanata L, Fontana G, Saibene F, Dicpinigaitis P, De Blasio F. Levodropropizine for treating cough in adult and children: a meta-analysis of published studies. Multidiscip Respir Med. 2015; 10:19.
3. Luporini G, Barni S, Marchi E, Daffonchio L. Efficacy and safety of levodropropizine and dihydrocodeine on nonproductive cough in primary and metastatic lung cancer. Eur Respir J. 1998; 12:97–101.
4. Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res. 2016; 8:541–544.
5. Baldo BA. IgE and drug allergy: antibody recognition of ‘small’ molecules of widely varying structures and activities. Antibodies (Basel). 2014; 3:56–91.
6. Ye YM, Kim MK, Kang HR, Kim TB, Sohn SW, Koh YI, et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res. 2015; 7:22–29.
7. Hur GY, Lee SY, Shim JJ, Park HS, Kang KH. An anaphylactic reaction caused by levodropropizine. Allergy. 2010; 65:409–410.
8. Yoon K, Kim SH, Ahn Y. A case of levodropropizine-induced anaphylaxis. Korean J Asthma Allergy Clin Immunol. 2011; 31:219–222.
9. Park KH, Yun IS, Choi SY, Lee JH, Hong CS, Park JW. A case of immunoglobulin E mediated anaphylaxis to levodropropizine. Yonsei Med J. 2013; 54:262–264.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download