Abstract
Periostin acts both as an extracellular matrix protein belonging to the fasciclin family and as a matricellular protein functioning in cell activation by binding to its receptors on the cell surface. It has been established that periostin is a downstream molecule of interleukin (IL)-13, a signature type 2 cytokine, and that periostin plays an important role in the pathogenesis of allergic diseases, including asthma. Based on these findings, much attention has been paid to periostin as a biomarker useful in the treatment of asthma. Periostin is a surrogate biomarker for type 2 immunity; it has been shown that serum periostin can predict the efficacy of anti-IL-13 antibodies (lebrikizumab) and anti-IgE antibodies (omalizumab), and that this usefulness can be potentially expanded to other type 2 antagonists. Moreover, it has been shown that periostin is not a simple surrogate biomarker for type 2 immunity; periostin-high asthma patients have several unique characteristics, including eosinophilia, high fraction of nitric oxide, aspirin intolerance, nasal disorders, and late onset. These characteristics are likely to be correlated with the involvement of periostin in the tissue remodeling of asthma. Periostin is also associated with hyporesponsiveness to inhaled corticosteroids, probably reflecting tissue remodeling. Thus, periostin has 2 characteristics as a biomarker for early diagnosis of asthma: surrogate biomarkers for type 2 immunity and tissue remodeling. Based on these characteristics, we will be able to apply serum periostin to treatment of asthma.
Periostin is an extracellular matrix (ECM) protein belonging to the fasciclin family, based on its homology to fasciclin 1 (FAS1).123 Periostin also acts as a matricellular protein that functions in cell activation by binding to its receptors, several integrins—αVβ1, αVβ3, αVβ5, α6β4, and αMβ2—on the cell surface. The actions of periostin as both an ECM protein and as a matricellular protein are important for the development and remodeling of many tissues, such as bone, heart, and skin.12 Moreover, it has been revealed that periostin plays an important role in allergic inflammation, including asthma.234 Based on these findings, much attention has been paid to periostin as a useful biomarker for treating asthma. In this review article, we focus on the latest findings on how best to do so. Regarding other topics about periostin—molecular characteristics, involvement in inflammatory mechanisms, association with diseases other than asthma, and its application to the development of therapeutic agents—please refer to other, recent review articles.1234
The importance of type 2 immunity in the pathogenesis of asthma was established in the 1990s, based on analyses of model mice.56 Thereafter, the research focus shifted to which signature cytokine in type 2 immunity—interleukin (IL)-4, IL-5, or IL-13—was important or to identifying the role of each of these cytokines in the pathogenesis of asthma. As it turns out, IL-13 plays a central role; it was shown that IL-13 alone was sufficient to cause asthma-like phenotypes in mice, whereas the blockage of IL-13 signals alone was sufficient to inhibit asthma-like phenotypes in ovalbumin-induced asthma model mice.78 Particularly in the pathogenesis of asthma, actions of IL-13 on airway epithelial cells have been shown to be important for inducing goblet cells and for enhancing airway hyper-responsiveness.910
To elucidate the effects of IL-13 on human airway epithelial cells, we comprehensively identified IL-13-inducible genes using the DNA microarray method.11 Consequently, we found that periostin is one of the highly expressed genes. IL-4, another cytokine sharing receptors and signal transduction pathways with IL-13, has the same ability to induce periostin.
We then investigated the expression of periostin in asthmatic patients using immunohistochemical analyses.1213 We found that periostin is deposited on the thickened basement membrane in asthmatic patients (Fig. 1). The localization of periostin overlapping with that of other ECM proteins composing thickened basement membrane—collagens I, III, and V, and tenascin-C—suggests that periostin contributes to generating subepithelial fibrosis in bronchial asthma by binding to other ECM proteins. Deposited periostin could be observed in the subepithelial areas of model mice in an IL-4- or IL-13-dependent manner.12 Woodruff et al.14 then confirmed that periostin is a gene highly expressed in the bronchial tissues of asthmatic patients. They showed that periostin expression by IL-13 is sensitive to corticosteroids and that expression of periostin is down-regulated with corticosteroid treatment in asthmatic patients.
The pathological role of periostin in asthma still remains controversial; several studies using periostin-deficient mice showed that periostin plays a protective role in airway allergic inflammation,1516 whereas another study with periostin-deficient mice and neutralizing antibodies against periostin showed that periostin accelerates it.17 The reason for this discrepancy is unclear. In contrast, Kanemitsu et al.18 followed up asthmatic patients for more than 20 years. They examined periostin expression in biopsy samples for more than 20 years ago and evaluated the change in FEV1. Consequently, it turned out that the more periostin was deposited in the lungs, the more pulmonary function decreased. Putting these findings together with our reports showing that periostin activates NF-κB by itself in keratinocytes and also activates NF-κB together with other inflammatory cytokines, such as TNFα or IL-1α in fibroblasts,1920 we assume that periostin exacerbates airway allergic inflammation.
It has been widely accepted that asthma is not a single disease, but rather a "syndrome."21 This concept is important for treating asthmatic patients, particularly for using molecularly targeted drugs. To support this concept, it is important to elucidate each "endotype" instead of each "phenotype" in heterogeneous subgroups comprising asthma.21 Although many trials have been performed to cluster asthmatic patients, the classification of asthmatic patients into "Th2-high" and "Th2-low" is important because it is potentially related to the choice of type 2 antagonists.21
Many molecularly targeted drugs against bronchial asthma are now under development. Approximately half of them are type 2 antagonists (Table 1). They target IgE, cytokines, chemokines, or prostaglandin D2 receptors, all of which are involved in type 2 immunity. If we are to prescribe these agents for asthmatic patients, we have to select patients in which type 2 immunity is dominant in their pathogenesis and for whom we can expect that type 2 antagonists would show efficacy. Establishment of such "stratified" medicines is necessary in using molecularly targeted drugs, both to increase their efficacy and to decrease costs.222324 Although it was initially reported that anti-IL-5 antibodies were not effective overall for asthmatic patients,2526 they later showed good efficacies for patients with high eosinophils.2728 Several agents targeting IL-4 or IL-13 did not show sufficient efficacy either, so their development was stopped,293031 which might have been due in part to not stratifying the patients.
Woodruff et al.32 stratified asthmatic patients into "Th2-high" and "Th2-low" based on the expression of IL-13 and IL-5. They then searched for signature molecules of "Th2-high" asthma, finding that 3 gene products—periostin, chloride channel regulator 1, and serpin peptidase inhibitor, clade B, member 2—correspond to these molecules, respectively.32
Based on this knowledge, Genentech33 applied serum periostin as a surrogate marker for Th2-high asthma and conducted a phase IIb study of anti-IL-13 antibodies (lebrikizumab) for steroid-resistant asthmatic patients. In this trial, lebrikizumab showed overall good efficacy in improving lung function for the patients. When the patients were divided into the high and low periostin groups based on serum periostin levels, lebrikizumab showed significant efficacy for the high periostin group, whereas it had no efficacy for the low periostin group. This study is a milestone in the field of asthma in that it showed for the first time that for asthma, periostin can be a target for a companion diagnostic, defined as one useful for predicting the efficacy of drugs following diagnosis.
Recently, it has been reported that clustering asthmatic patients into the high and low periostin groups is useful for predicting the efficacy of anti-IgE antibodies (omalizumab) as well.34 It is noteworthy that the target molecules of anti–IL-13 antibodies and anti-IgE antibodies are different; however, both IL-13 and IgE are type 2 immunity-related molecules, so serum periostin predicts the efficacy of both agents. Thus, periostin would be a surrogate biomarker for not only IL-13, but also type 2 immunity. Based on these findings, an algorithm for the treatment of asthma can be proposed (Fig. 2). The first line of anti-asthma drugs is inhaled corticosteroids (ICSs). Although ICSs are effective for most patients, 5%-10% are resistant or hyporesponsive to them.3536 Measurement of serum periostin is recommended for these patients. If some patients show high periostin levels, type 2 antagonists, such as lebrikizumab or omalizumab, should be added. If some show low periostin levels, since type 2 antagonists would be ineffective, other agents or therapies is recommended. However, the story is not so simple. Other biomarkers—eosinophils, fraction of nitric oxide (FeNO), and dipeptidyl peptidase-4 (DPP4)—are also used as surrogate biomarkers for type 2 immunity and as possible companion diagnostics for type 2 antagonists (Table 2). It remains to be addressed what these biomarkers have in common and how they differ, as well as which biomarker or combination is optimal for predicting the efficacy of each type 2 antagonist. Moreover, periostin is not a simple surrogate biomarker for type 2 immunity, as shown in the next section.
As mentioned earlier, asthma is a heterogeneous disease, and many trials have been reported for clustering asthmatic patients.3738 Serum periostin levels are diverse among asthmatic patients; Kanemitsu et al.18 reported that 37.9% of 224 asthmatic patients receiving ICS treatment showed higher periostin levels than the normal range (<95 ng/mL), whereas the rest remained within the normal range. Although periostin has appeared as a surrogate biomarker for type 2 immunity, it has not been simple to characterize it. Several studies have attempted to clarify the characteristics of periostin-high asthmatic patients.
As far as we have seen, serum periostin reproducibly shows good correlations with blood or sputum eosinophilia.18394041 IL-5 and IL-13, key cytokines for the induction of eosinophilia and the production of periostin, respectively, are both signature type 2 cytokines, which may explain the good correlation between serum periostin and eosinophils. Previous studies failed to detect this correlation,42 which may be explained by the differences in the detection system for periostin.43
Nasal disorders, such as chronic sinusitis, nasal polyps, olfactory dysfunction, and allergic rhinitis, are common comorbidities of asthma. Particularly, chronic sinusitis with nasal polyps is accompanied by aspirin-induced asthma. Serum periostin is well correlated with nasal disorders.18394041 This finding is consistent with periostin being highly expressed in the lesions of patients with chronic sinusitis.45
Serum periostin is also well correlated with late-onset asthma.184041 Bobolea et al.46 showed a good correlation of sputum periostin with late-onset asthma. It is generally known that late-onset asthma is eosinophil-dominant and is more often non-atopic than childhood asthma.47 It is noteworthy hat late-onset asthmatic patients show a more rapid lung dysfunction, a lower remission rate, and a poorer prognosis. However, late-onset asthma has some heterogeneity.48 Haldar et al.37 and Moore et al.38 reported 2 different late-onset asthma types: (1) obesity and female sex type and (2) active airway inflammation, fixed airflow limitation, male sex, and longer duration type. The active airway inflammation, fixed airflow limitation, male sex, and longer duration type would correspond to the high-periostin type, whereas the obesity and female sex type would not.
These findings suggest that periostin is not just a surrogate biomarker for type 2 immunity, but a biomarker that picks up some specific subgroup in "Th2-high" asthma. Moreover, these findings would help clarify the underlying mechanism of the efficacy of type 2 antagonists.
Tissue remodeling of bronchial tissues, including fibrosis, is a typical histological characteristic of asthma. On the other hand, although ICSs are powerful and effective drugs for asthmatic patients and are used as a first-line drug for asthma, 5%-10% of asthmatic patients are resistant or hyporesponsive to them.35 It is assumed that hyporesponsiveness to ICSs is caused by many underlying mechanisms, such as tissue remodeling.49 Given that periostin is a component of fibrosis, a feature of tissue remodeling, it is reasonable to suppose that serum periostin could be a biomarker to predict hyporesponsiveness to ICSs.
Kanemitsu et al.18 evaluated hyporesponsiveness to ICSs by decline of FEV1 (ΔFEV1) in the course of treatment with ICSs; Kanemitsu et al. reported that serum periostin is correlated with the decline FEV1 in overall asthmatic patients. When the patients were divided into the rapid decliners (ΔFEV1 ≤-30 mL/year) and non-rapid decliners (ΔFEV1 >-30 mL/year), the rapid decliners showed a higher periostin level than the non-rapid decliners (104.6 vs 89.2 ng/mL), suggesting that periostin is a surrogate biomarker for ICS hyporesponsiveness. However, the difference in the averages of periostin levels between the rapid and non-rapid decliners was not significant, which may be attributed to the heterogeneous mechanisms of hyporesponsiveness to ICSs.
Nagasaki et al.50 explored the possibility that if asthmatic patients are clustered into several groups, some groups may show better correlation between serum periostin and hyporesponsiveness to ICSs than the overall groups. They applied blood eosinophils and neutrophils for clustering patients, finding that the patients could be subdivided into 4 clusters: Cluster 1 (low eosinophils and low neutrophils), late-onset and non-atopic; Cluster 2 (moderate eosinophils and low neutrophils), early-onset and atopic; Cluster 3 (high eosinophils and low neutrophils), late-onset and eosinophil-dominance; and Cluster 4 (moderate eosinophils and high neutrophils), poor control and high IL-6. The patients in Clusters 1 and 2 were good responders to ICSs irrespective of serum periostin, whereas the patients in Cluster 4 were poor responders, irrespective of serum periostin. It is noteworthy that the difference in ΔFEV1 between the high periostin (>95 ng/mL) and low periostin (≤95 ng/mL) groups is significant in Cluster 3 (-23.0 vs -1.42 mL/year). As mentioned earlier, Cluster 3 would greatly overlap with the subgroup manifesting active airway inflammation, fixed airflow limitation, male sex, and longer duration, as reported by Haldar et al.37 and Moore et al.38 These results suggest the ability of serum periostin to predict the hyporesponsiveness to ICSs, particularly in Cluster 3 patients, namely the patients with adult-onset and eosinophil-dominant asthma.
Kato et al.51 examined the ability of serum periostin to predict the hyporesponsiveness to ICSs from a different point of view. They enrolled 25 asthmatic patients well controlled by ICSs. They observed the patients for 12 weeks after they tapered off ICSs and divided them into the stable (n=20) and unstable (n=5) groups, based on the occurrence of acute exacerbation. When they compared serum periostin levels in the patients before tapering ICSs, the unstable group showed a higher periostin level than the stable group (141.9 vs 91.5 ng/mL). These results suggest that high periostin levels entail the risk of acute exacerbation by tapering ICSs. Although examined subjects and endpoints are different among the above studies, serum periostin appears to be a good biomarker in patients who cannot maintain lung functions with ICSs, and it can also be a good biomarker in patients who will not be able to taper off ICS treatment when they are stable.
In contrast to adult asthma, the usefulness of periostin in childhood asthma is still under discussion. Song et al.52 demonstrated high periostin levels in patients with childhood asthma; however, the difference between the patients and control subjects was subtle (76.0 vs 71.0 ng/mL). They also showed the correlation of serum periostin with other type 2 immunity biomarkers, blood eosinophils, and FeNO, as in adult asthma. In contrast, Konradsen et al.53 did not detect correlations of serum periostin with blood eosinophils or FeNO in childhood asthma patients. Moreover, Inoue et al.54 did not find any difference in serum periostin levels between patients with childhood asthma and control subjects. One reason for the inconsistent results with serum periostin levels in patients with childhood asthma could be high baseline levels of serum periostin in children. Inoue et al.54 showed that serum periostin levels in elementary school-age children were higher than adults (6-11 years; mean: 125.0 ng/mL) and become higher as the children grew older (Fig. 3). High serum periostin levels in the teenagers dropped after puberty. This was probably because serum periostin in teens is mostly derived from bones and thereafter drops because bone growth stops. Another reason could be that there is less tissue remodeling in childhood asthma than in adult asthma. As mentioned earlier, serum periostin has characteristics as a surrogate biomarker for tissue remodeling in addition to type 2 immunity. Although the pathogenesis of childhood asthma is mostly type 2 immunity, type 2 immunity may not be sufficient to enhance serum periostin as in adult asthma. The finding that adult patients with early-onset and atopic type of asthma do not show high periostin levels as those with late-onset type asthma may support this idea.50 Moreover, it is noteworthy that we should be careful in evaluating serum periostin levels in children because normal ranges vary among different age groups.
Periostin has appeared as a novel surrogate biomarker for type 2 immunity in asthmatic patients. However, periostin is not a straightforward biomarker for type 2 immunity, but has several unique characteristics. These characteristics are likely to be correlated with the tissue remodeling of asthma. Based on the above findings, periostin is useful for dissecting endotypes in asthma, including hyporesponsiveness to ICSs. In turn, these findings would be useful for understanding the underlying mechanism of the efficacy of type 2 antagonists. Moreover, it is important to clarify common and different characteristics between periostin and other surrogate biomarkers, such as eosinophils, FeNO, and DPP4. The ability to select the optimal biomarker or a combination of biomarkers is required as soon as possible if we are to successfully apply type 2 antagonists for asthma.
Figures and Tables
Fig. 1
Expression of periostin in asthmatic patients.12 The histochemical localization of periostin in asthmatic patients is depicted. The left and right panels show bronchial tissues from an asthmatic patient in H&E staining (A) and immunostaining (B) of periostin. Periostin in the right panel is stained brown and is localized in the thickened basement membrane in asthmatic patients.
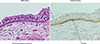
Fig. 2
Algorithm for the treatment of asthma. A first-line antiasthma drug is ICSs. If a patient is resistant or hyporesponsive to ICSs, serum periostin should be measured If a patient shows a high periostin level, type 2 antagonists should be added. If a patient shows a low periostin level, other agents or therapies are recommended.

Fig. 3
Serum periostin levels according to age.54 (A) Serum periostin levels in the elementary school-age children without allergic diseases. Bottom of the box, 25th percentile; Line in the middle of box, median; Top of the box, 75th percentile; Whiskers, to the smallest value and to the largest value. (B) The serum periostin levels of subjects without allergic diseases in both elementary school-age children and adults.

Table 1
Type 2 antagonists against bronchial asthma under development

Table 2
Biomarkers for the application of type 2 antagonists
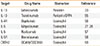
| Target | Drug Name | Biomarker | Reference |
|---|---|---|---|
| IL-13 | Lebrikizumab | Periostin | 33 |
| IL-13 | Tralokinumab | Periostin/DPP4 | 55 |
| IL-4R | Dupilumab | Eosinophil | 56 |
| IL-5 | Mepolizumab | Eosinophil | 27, 28 |
| IL-5 | Reslizumab | Eosinophil | 57 |
| IL-5R | Benralizumab | Eosinophil | 58 |
| CRTH2 | OC459/ODC9101 | Eosinophil | 59 |
ACKNOWLEDGMENTS
We thank Dr. Dovie R. Wylie for the critical review of this manuscript. This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
References
1. Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014; 71:1279–1288.
2. Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014; 63:143–151.
3. Izuhara K, Matsumoto H, Ohta S, Ono J, Arima K, Ogawa M. Recent developments regarding periostin in bronchial asthma. Allergol Int. 2015; 64:Suppl. S3–S10.
4. Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. Forthcoming 2016.
5. Izuhara K, Arima K, Kanaji S, Ohta S, Kanaji T. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006; 13:2291–2298.
6. Izuhara K, Ohta S, Shiraishi H, Suzuki S. Interleukin 4, interleukin 13, and interleukin 9. In : Izuhara K, Holgate ST, Wills-Karp M, editors. Inflammation and allergy drug design. London: Wiley-Blackwell;2011. p. 175–185.
7. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263.
8. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282:2258–2261.
9. Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999; 103:779–788.
10. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002; 8:885–889.
11. Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002; 19:287–296.
12. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; 118:98–104.
13. Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-γ and IL-13 production. Proc Natl Acad Sci U S A. 2007; 104:14765–14770.
14. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863.
15. Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011; 186:4959–4966.
16. Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, et al. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012; 42:144–155.
17. Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, et al. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014; 134:1433–1442.
18. Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013; 132:305–312.e3.
19. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012; 122:2590–2600.
20. Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012; 46:677–686.
21. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.
22. Willis JC, Lord GM. Immune biomarkers: the promises and pitfalls of personalized medicine. Nat Rev Immunol. 2015; 15:323–329.
23. Hughes B. Developing tools for stratified medicine. Nat Rev Drug Discov. 2009; 8:919–920.
24. Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007; 6:287–293.
25. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.
26. Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003; 167:1655–1659.
27. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–1207.
28. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.
29. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431.
30. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010; 181:788–796.
31. Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011; 183:1007–1014.
32. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.
33. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.
34. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.
35. Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003; 178:347–355.
36. Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985). 2008; 104:394–403.
37. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008; 178:218–224.
38. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.
39. Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014; 113:314–320.
40. Matsusaka M, Kabata H, Fukunaga K, Suzuki Y, Masaki K, Mochimaru T, et al. Phenotype of asthma related with high serum periostin levels. Allergol Int. 2015; 64:175–180.
41. Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. Forthcoming 2016.
42. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015; 70:115–120.
43. Arron JR, Izuhara K. Asthma biomarkers: what constitutes a 'gold standard'? Thorax. 2015; 70:105–107.
44. Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Horiguchi T, et al. Using exhaled nitric oxide and serum periostin as a composite marker to identify severe/steroid-insensitive asthma. Am J Respir Crit Care Med. 2014; 190:1449–1452.
45. Ishida A, Ohta N, Suzuki Y, Kakehata S, Okubo K, Ikeda H, et al. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol Int. 2012; 61:589–595.
46. Bobolea I, Barranco P, Del Pozo V, Romero D, Sanz V, López-Carrasco V, et al. Sputum periostin in patients with different severe asthma phenotypes. Allergy. 2015; 70:540–546.
47. Hekking PP, Bel EH. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract. 2014; 2:671–680.
48. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005; 172:149–160.
49. Kanemitsu Y, Matsumoto H, Mishima M. KiHAC Respiratory Medicine Group. Factors contributing to an accelerated decline in pulmonary function in asthma. Allergol Int. 2014; 63:181–188.
50. Nagasaki T, Matsumoto H, Kanemitsu Y, Izuhara K, Tohda Y, Kita H, et al. Integrating longitudinal information on pulmonary function and inflammation using asthma phenotypes. J Allergy Clin Immunol. 2014; 133:1474–1477. 1477.e1–1477.e2.
51. Kato G, Takahashi K, Izuhara K, Komiya K, Kimura S, Hayashi S. Markers that can reflect asthmatic activity before and after reduction of inhaled corticosteroids: a pilot study. Biomark Insights. 2013; 8:97–105.
52. Song JS, You JS, Jeong SI, Yang S, Hwang IT, Im YG, et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy. 2015; 70:674–681.
53. Konradsen JR, Skantz E, Nordlund B, Lidegran M, James A, Ono J, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol. 2015; 26:772–779.
54. Inoue Y, Izuhara K, Ohta S, Ono J, Shimojo N. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol Int. 2015; 64:289–290.
55. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015; 3:692–701.
56. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.
57. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366.
58. Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879–890.
59. Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014; 69:1223–1232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download